Kymera Investor Presentation Deck
Mean (± SE) Percent IRAK4
Change from Baseline
50
-20
-40
-60
-80
-100
●
●
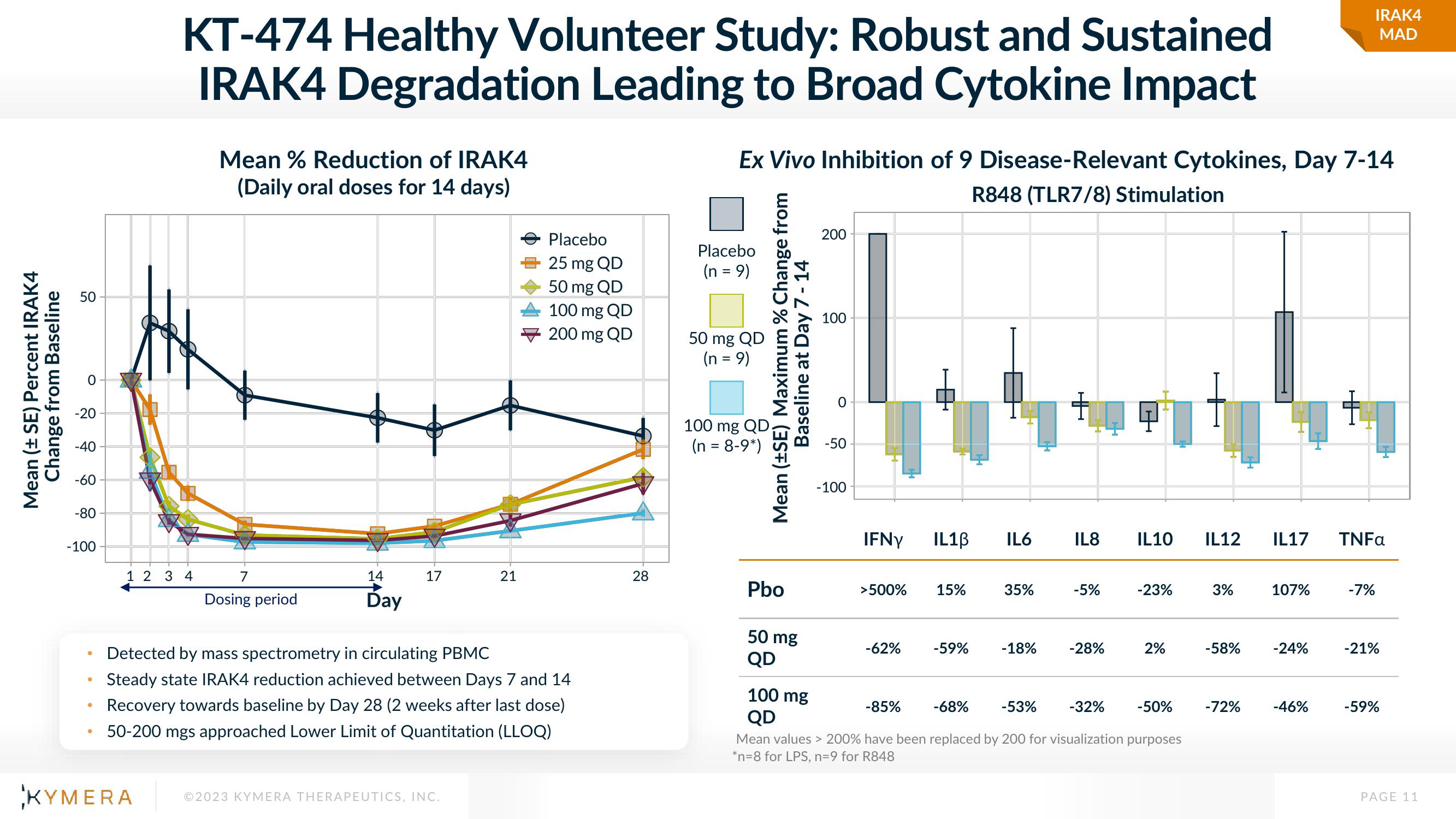
KT-474 Healthy Volunteer Study: Robust and Sustained
IRAK4 Degradation Leading to Broad Cytokine Impact
1 2 3 4
Mean % Reduction of IRAK4
(Daily oral doses for 14 days)
7
Dosing period
14
Day
17
21
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
Placebo
25 mg QD
50 mg QD
100 mg QD
200 mg QD
Detected by mass spectrometry in circulating PBMC
Steady state IRAK4 reduction achieved between Days 7 and 14
Recovery towards baseline by Day 28 (2 weeks after last dose)
50-200 mgs approached Lower Limit of Quantitation (LLOQ)
28
Ex Vivo Inhibition of 9 Disease-Relevant Cytokines, Day 7-14
R848 (TLR7/8) Stimulation
Placebo
(n = 9)
50 mg QD
(n = 9)
100 mg QD
(n = 8-9*)
Pbo
50 mg
QD
200
100
-50
-100
IFNY IL10 IL6
>500%
-62%
15%
-85%
-59%
35%
-18%
IL8 IL10 IL12
-53%
-5%
-28%
-23%
100 mg
-68%
QD
Mean values > 200% have been replaced by 200 for visualization purposes
*n=8 for LPS, n=9 for R848
2%
-32% -50%
3%
-58%
-72%
IL17
107%
-24%
-46%
IRAK4
MAD
TNFa
-7%
-21%
-59%
PAGE 11View entire presentation