Kymera Investor Day Presentation Deck
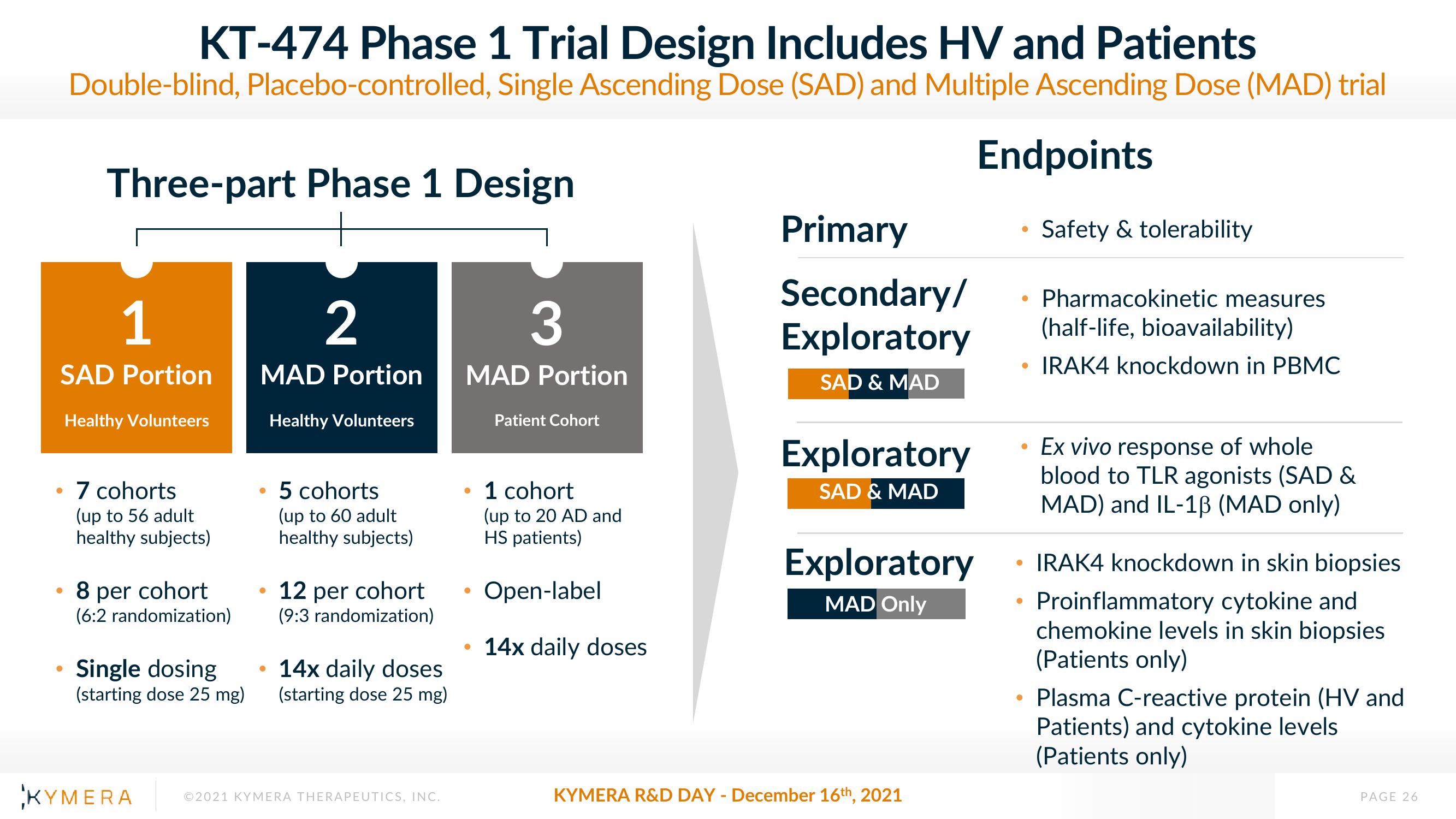
KT-474 Phase 1 Trial Design Includes HV and Patients
Double-blind, placebo-controlled, Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) trial
Endpoints
Three-part Phase 1 Design
1
SAD Portion
Healthy Volunteers
• 7 cohorts
(up to 56 adult
healthy subjects)
8 per cohort
(6:2 randomization)
Single dosing
(starting dose 25 mg)
2
MAD Portion
Healthy Volunteers
5 cohorts
(up to 60 adult
healthy subjects)
12 per cohort
(9:3 randomization)
• 14x daily doses
(starting dose 25 mg)
KYMERA ©2021 KYMERA THERAPEUTICS, INC.
3
MAD Portion
Patient Cohort
• 1 cohort
(up to 20 AD and
HS patients)
Open-label
14x daily doses
Primary
Secondary/
Exploratory
SAD & MAD
Exploratory
SAD & MAD
Exploratory
MAD Only
KYMERA R&D DAY - December 16th, 2021
●
●
Pharmacokinetic measures
(half-life, bioavailability)
• IRAK4 knockdown in PBMC
●
●
Safety & tolerability
●
Ex vivo response of whole
blood to TLR agonists (SAD &
MAD) and IL-1B (MAD only)
IRAK4 knockdown in skin biopsies
Proinflammatory cytokine and
chemokine levels in skin biopsies
(Patients only)
Plasma C-reactive protein (HV and
Patients) and cytokine levels
(Patients only)
PAGE 26View entire presentation