Acquisition of APIRx
For personal use only
CanChew Rx and SuppoCan for
Inflammatory Bowel Disease
Problem
68 million people suffer from Inflammatory
Bowel Disease globally. Signs and symptoms
of both Crohn's disease and ulcerative colitis
include diarrhea, fatigue, and abdominal pain
and cramping, reduced appetite, and unintended
weight loss. Heretofore, the main medications for
IBD are anti-inflammatory medications
and analgesics.
Solution
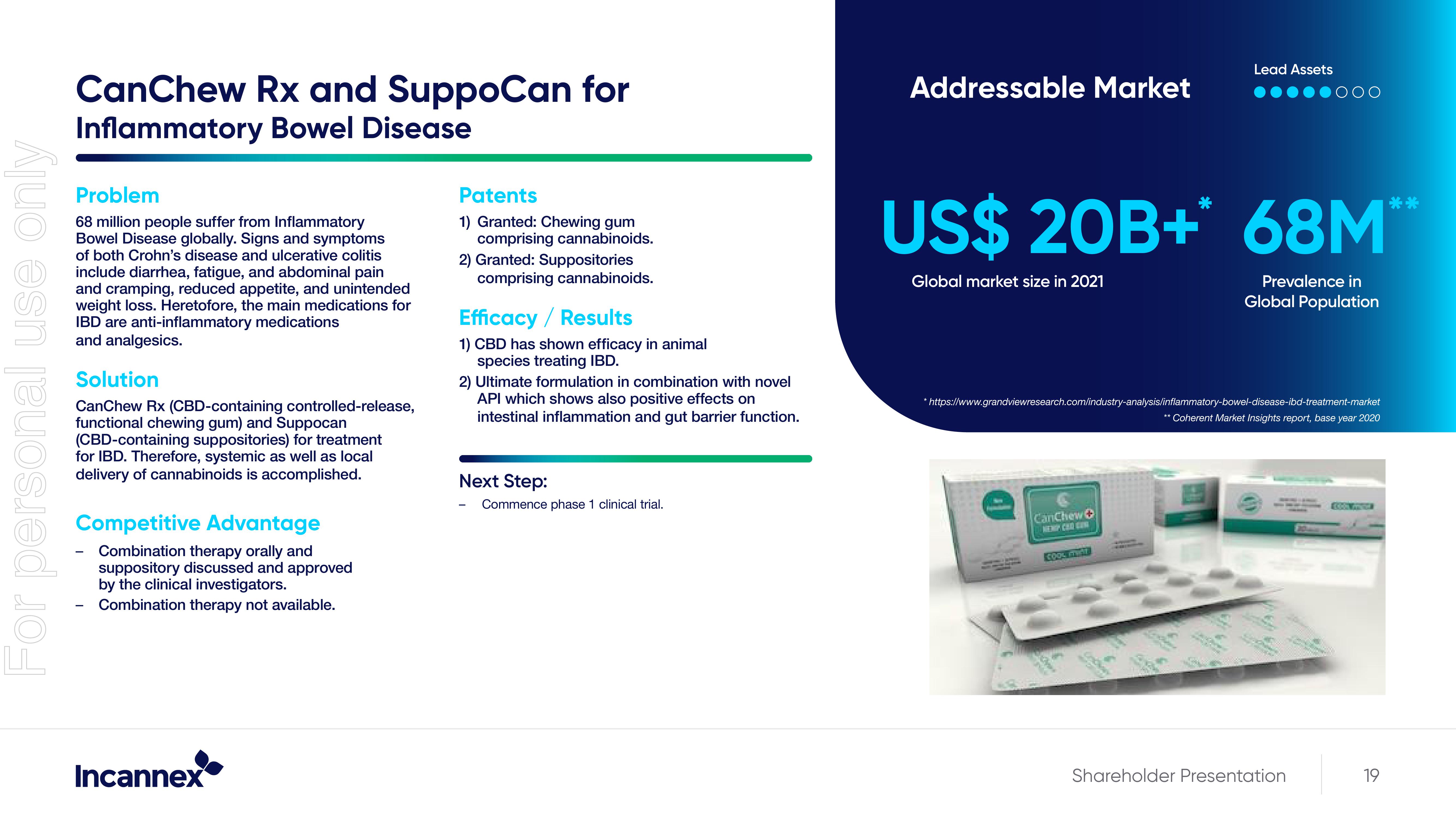
CanChew Rx (CBD-containing controlled-release,
functional chewing gum) and Suppocan
(CBD-containing suppositories) for treatment
for IBD. Therefore, systemic as well as local
delivery of cannabinoids is accomplished.
Competitive Advantage
Combination therapy orally and
suppository discussed and approved
by the clinical investigators.
Combination therapy not available.
Incannex
Patents
1) Granted: Chewing gum
comprising cannabinoids.
2) Granted: Suppositories
comprising cannabinoids.
Efficacy/Results
1) CBD has shown efficacy in animal
species treating IBD.
2) Ultimate formulation in combination with novel
API which shows also positive effects on
intestinal inflammation and gut barrier function.
Next Step:
Commence phase 1 clinical trial.
Addressable Market
US$ 20B+* 68M™*
Global market size in 2021
CanChew
NEMP CHO GUN
*https://www.grandviewresearch.com/industry-analysis/inflammatory-bowel-disease-ibd-treatment-market
** Coherent Market Insights report, base year 2020
COOL WA
231
KO
Lead Assets
SANA
CAN
Prevalence in
Global Population
Shareholder Presentation
19View entire presentation