Immix Biopharma Investor Presentation Deck
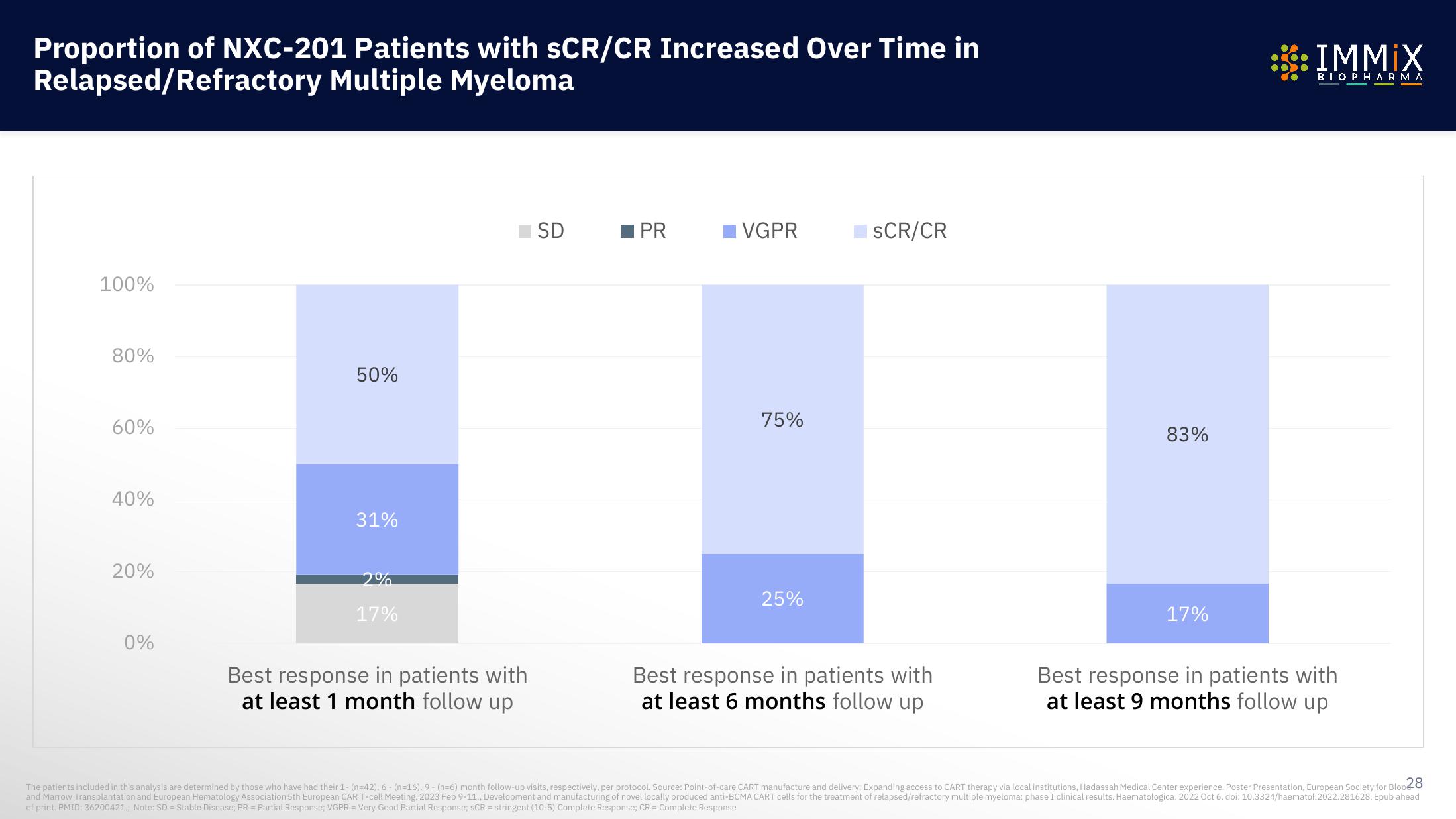
Proportion of NXC-201 Patients with SCR/CR Increased Over Time in
Relapsed/Refractory Multiple Myeloma
100%
80%
60%
40%
20%
0%
50%
31%
2%
17%
■
Best response in patients with
at least 1 month follow up
SD
PR
VGPR
75%
25%
SCR/CR
Best response in patients with
at least 6 months follow up
83%
17%
●●●
IMMIX
S BIOPHARMA
Best response in patients with
at least 9 months follow up
The patients included in this analysis are determined by those who have had their 1- (n=42), 6 - (n=16), 9 - (n=6) month follow-up visits, respectively, per protocol. Source: Point-of-care CART manufacture and delivery: Expanding access to CART therapy via local institutions, Hadassah Medical Center experience. Poster Presentation, European Society for Bloo28
and Marrow Transplantation and European Hematology Association 5th European CAR T-cell Meeting. 2023 Feb 9-11., Development and manufacturing of novel locally produced anti-BCMA CART cells for the treatment of relapsed/refractory multiple myeloma: phase I clinical results. Haematologica. 2022 Oct 6. doi: 10.3324/haematol.2022.281628. Epub ahead
of print. PMID: 36200421., Note: SD = Stable Disease; PR = Partial Response; VGPR = Very Good Partial Response; SCR = stringent (10-5) Complete Response; CR = Complete ResponseView entire presentation