Equillium Results Presentation Deck
Subject #: Baseline UPCR
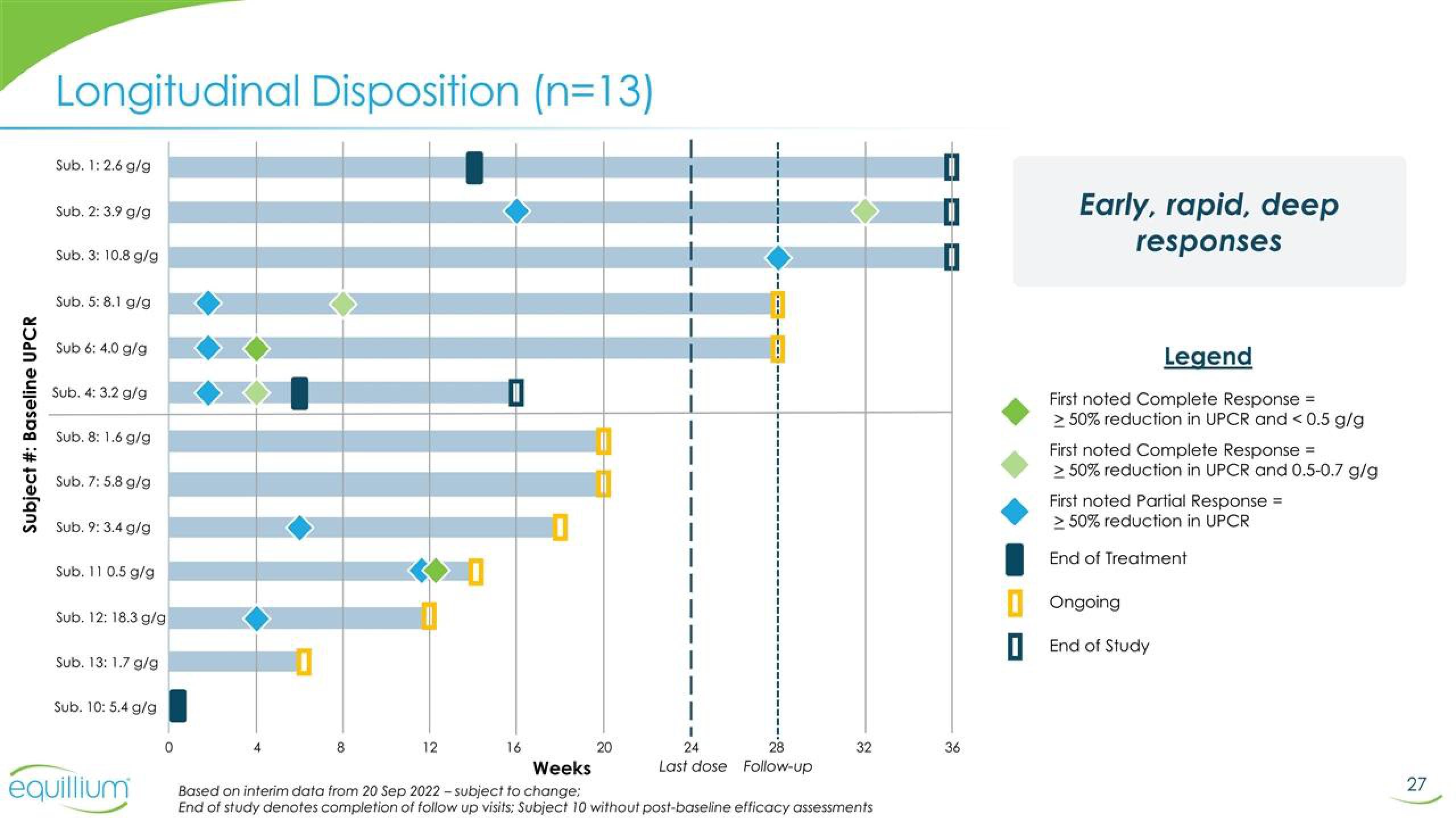
Longitudinal Disposition (n=13)
Sub. 1: 2.6 g/g
Sub. 2: 3.9 g/g
Sub. 3: 10.8 g/g
Sub. 5: 8.1 g/g
Sub 6: 4.0 g/g
Sub. 4: 3.2 g/g
Sub. 8: 1.6 g/g
Sub. 7: 5.8 g/g
Sub. 9: 3.4 g/g
Sub. 11 0.5 g/g
Sub. 12: 18.3 g/g
Sub. 13: 1.7 g/g
Sub. 10: 5.4 g/g
equillium
8
12
16
20
1
|
28
24
Last dose Follow-up
32
Weeks
Based on interim data from 20 Sep 2022-subject to change;
End of study denotes completion of follow up visits: Subject 10 without post-baseline efficacy assessments
36
Early, rapid, deep
responses
Legend
First noted Complete Response =
> 50% reduction in UPCR and < 0.5 g/g
First noted Complete Response
≥ 50% reduction in UPCR and 0.5-0.7 g/g
First noted Partial Response =
> 50% reduction in UPCR
End of Treatment
Ongoing
End of Study
=
27View entire presentation