Bionomics Investor Presentation Deck
BNC210 Phase 2 PREVAIL Social Anxiety Disorder Trial
Acute Social Anxiety Disorder Study Highlights
✓ Cost-effective trial with an efficacy endpoint
conducive to rapid data generation
✓ Ability to leverage VistaGen's development
plan and trial design for Social Anxiety
Disorder
Received FDA clearance for IND filing and
FDA Fast Track designation
✓ Phase 2 trial underway and will read out
topline data by end of 2022
Bionomics
LSAS = Liebowitz Social Anxiety Scale
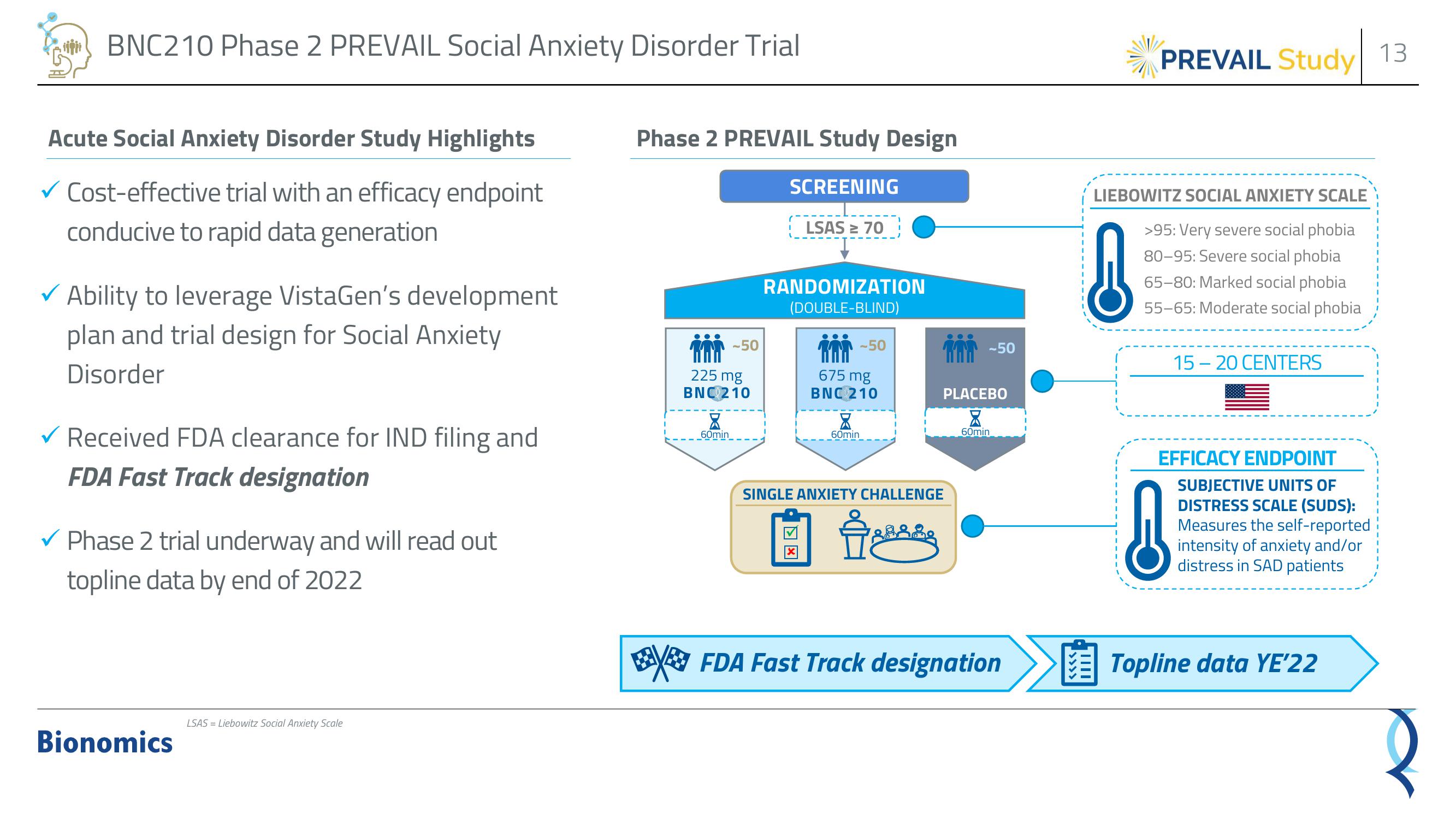
Phase 2 PREVAIL Study Design
-50
225 mg
BNC 210
60min
SCREENING
LSAS ≥ 70
RANDOMIZATION
(DOUBLE-BLIND)
-50
675 mg
BNC 210
60min
SINGLE ANXIETY CHALLENGE
台
frases
-50
PLACEBO
X
60min
FDA Fast Track designation
PREVAIL Study 13
LIEBOWITZ SOCIAL ANXIETY SCALE
>95: Very severe social phobia
80-95: Severe social phobia
65-80: Marked social phobia
55-65: Moderate social phobia
15-20 CENTERS
EFFICACY ENDPOINT
SUBJECTIVE UNITS OF
DISTRESS SCALE (SUDS):
Measures the self-reported
intensity of anxiety and/or
distress in SAD patients
Topline data YE'22View entire presentation