Conference Presentation
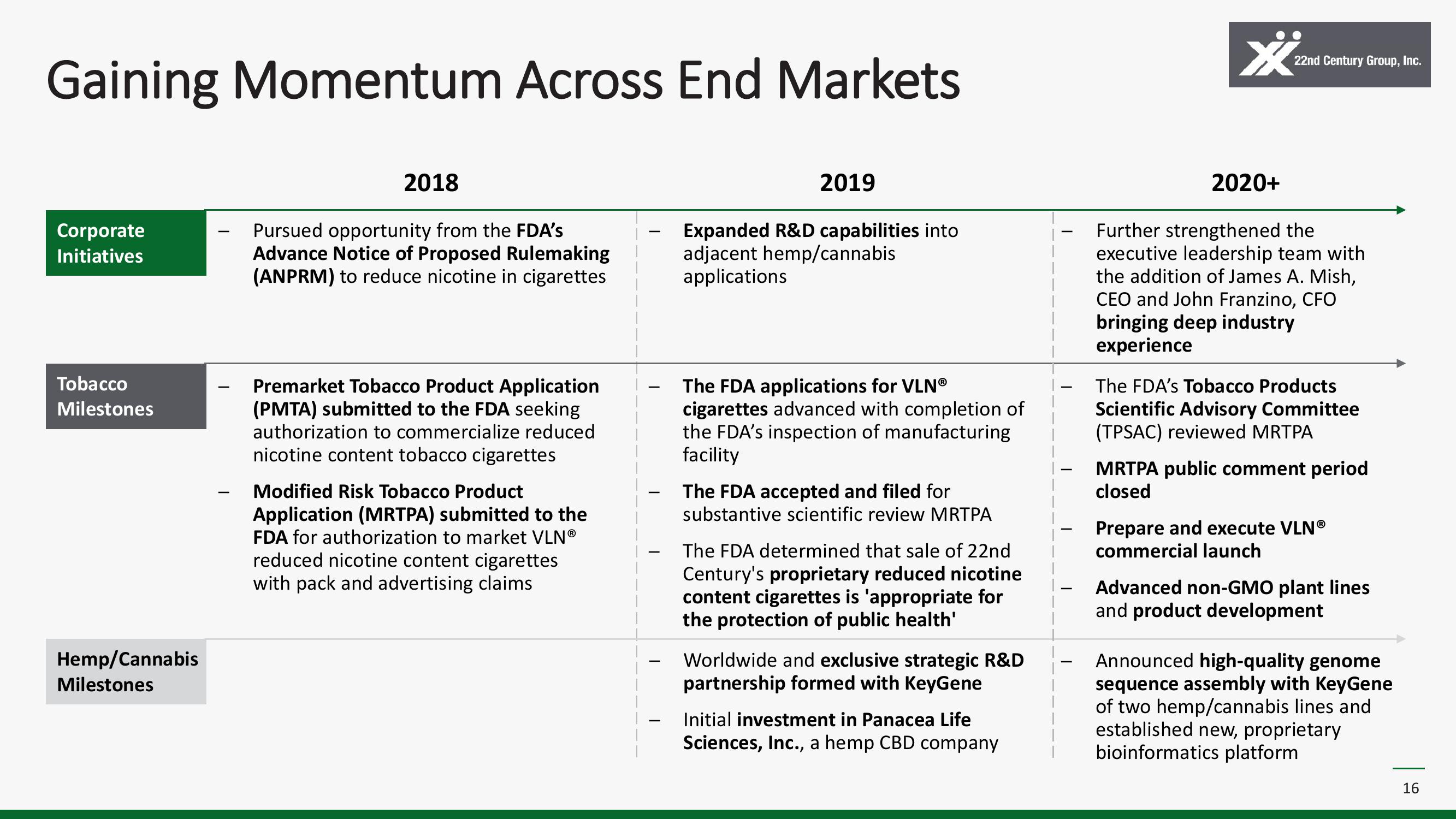
Gaining Momentum Across End Markets
Corporate
Initiatives
Tobacco
Milestones
Hemp/Cannabis
Milestones
2018
Pursued opportunity from the FDA's
Advance Notice of Proposed Rulemaking
(ANPRM) to reduce nicotine in cigarettes
Premarket Tobacco Product Application
(PMTA) submitted to the FDA seeking
authorization to commercialize reduced
nicotine content tobacco cigarettes
Modified Risk Tobacco Product
Application (MRTPA) submitted to the
FDA for authorization to market VLNⓇ
reduced nicotine content cigarettes
with pack and advertising claims
|
2019
Expanded R&D capabilities into
adjacent hemp/cannabis
applications
The FDA applications for VLNⓇ
cigarettes advanced with completion of
the FDA's inspection of manufacturing
facility
The FDA accepted and filed for
substantive scientific review MRTPA
The FDA determined that sale of 22nd
Century's proprietary reduced nicotine
content cigarettes is 'appropriate for
the protection of public health'
Worldwide and exclusive strategic R&D
partnership formed with KeyGene
Initial investment in Panacea Life
Sciences, Inc., a hemp CBD company
*
2020+
22nd Century Group, Inc.
Further strengthened the
executive leadership team with
the addition of James A. Mish,
CEO and John Franzino, CFO
bringing deep industry
experience
The FDA's Tobacco Products
Scientific Advisory Committee
(TPSAC) reviewed MRTPA
MRTPA public comment period
closed
Prepare and execute VLNⓇ
commercial launch
Advanced non-GMO plant lines
and product development
Announced high-quality genome
sequence assembly with KeyGene
of two hemp/cannabis lines and
established new, proprietary
bioinformatics platform
16View entire presentation