Ocuphire Pharma Investor Day Presentation Deck
RM
66
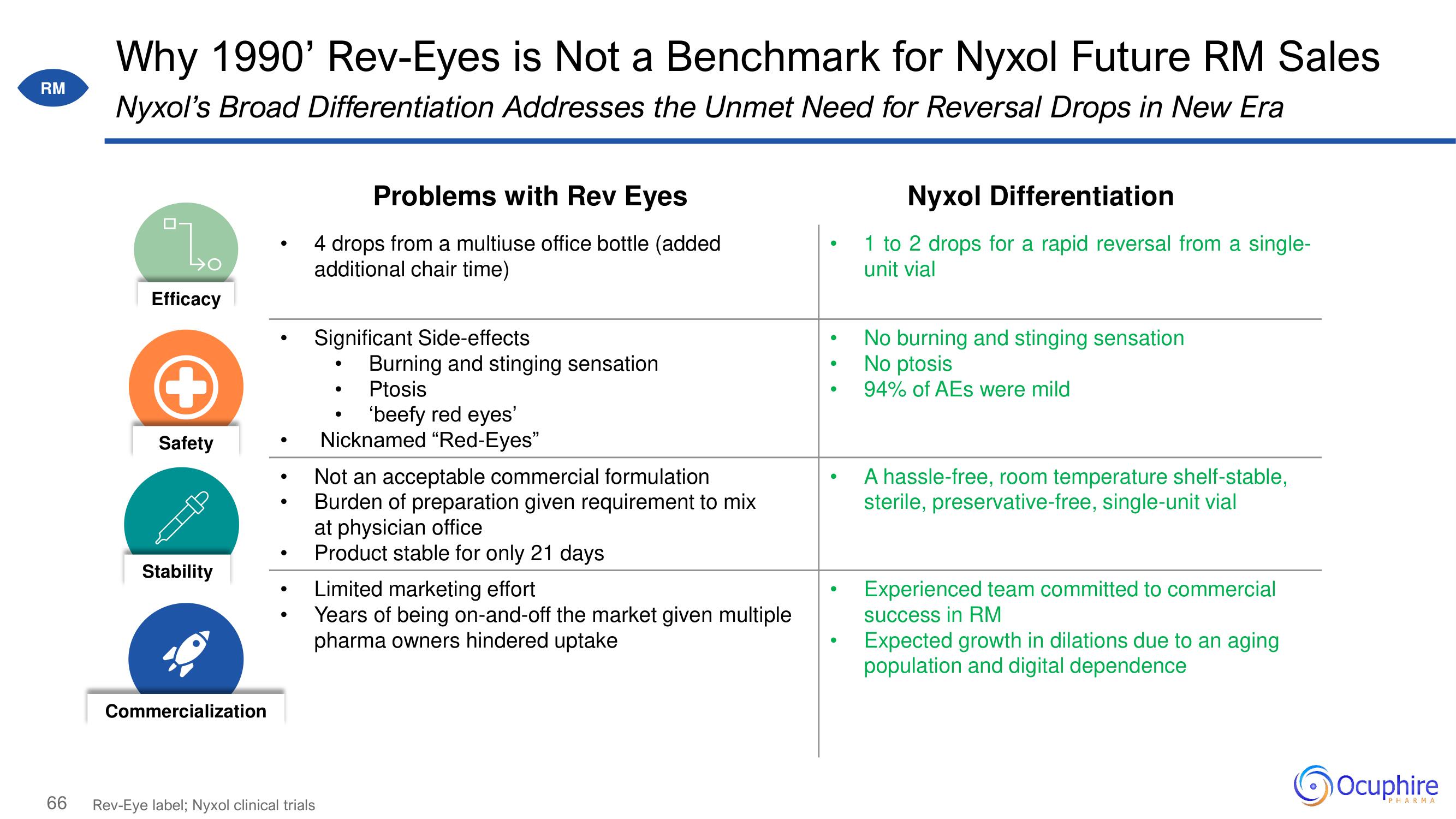
Why 1990' Rev-Eyes is Not a Benchmark for Nyxol Future RM Sales
Nyxol's Broad Differentiation Addresses the Unmet Need for Reversal Drops in New Era
010
Efficacy
Safety
Stability
Commercialization
●
●
●
●
●
Problems with Rev Eyes
4 drops from a multiuse office bottle (added
additional chair time)
Significant Side-effects
●
●
Rev-Eye label; Nyxol clinical trials
Burning and stinging sensation
Ptosis
'beefy red eyes'
Nicknamed "Red-Eyes"
Not an acceptable commercial formulation
Burden of preparation given requirement to mix
at physician office
Product stable for only 21 days
Limited marketing effort
Years of being on-and-off the market given multiple
pharma owners hindered uptake
●
Nyxol Differentiation
1 to 2 drops for a rapid reversal from a single-
unit vial
No burning and stinging sensation
No ptosis
94% of AEs were mild
A hassle-free, room temperature shelf-stable,
sterile, preservative-free, single-unit vial
Experienced team committed to commercial
success in RM
Expected growth in dilations due to an aging
population and digital dependence
Ocuphire
PHARMAView entire presentation