Dare Bioscience Investor Presentation Deck
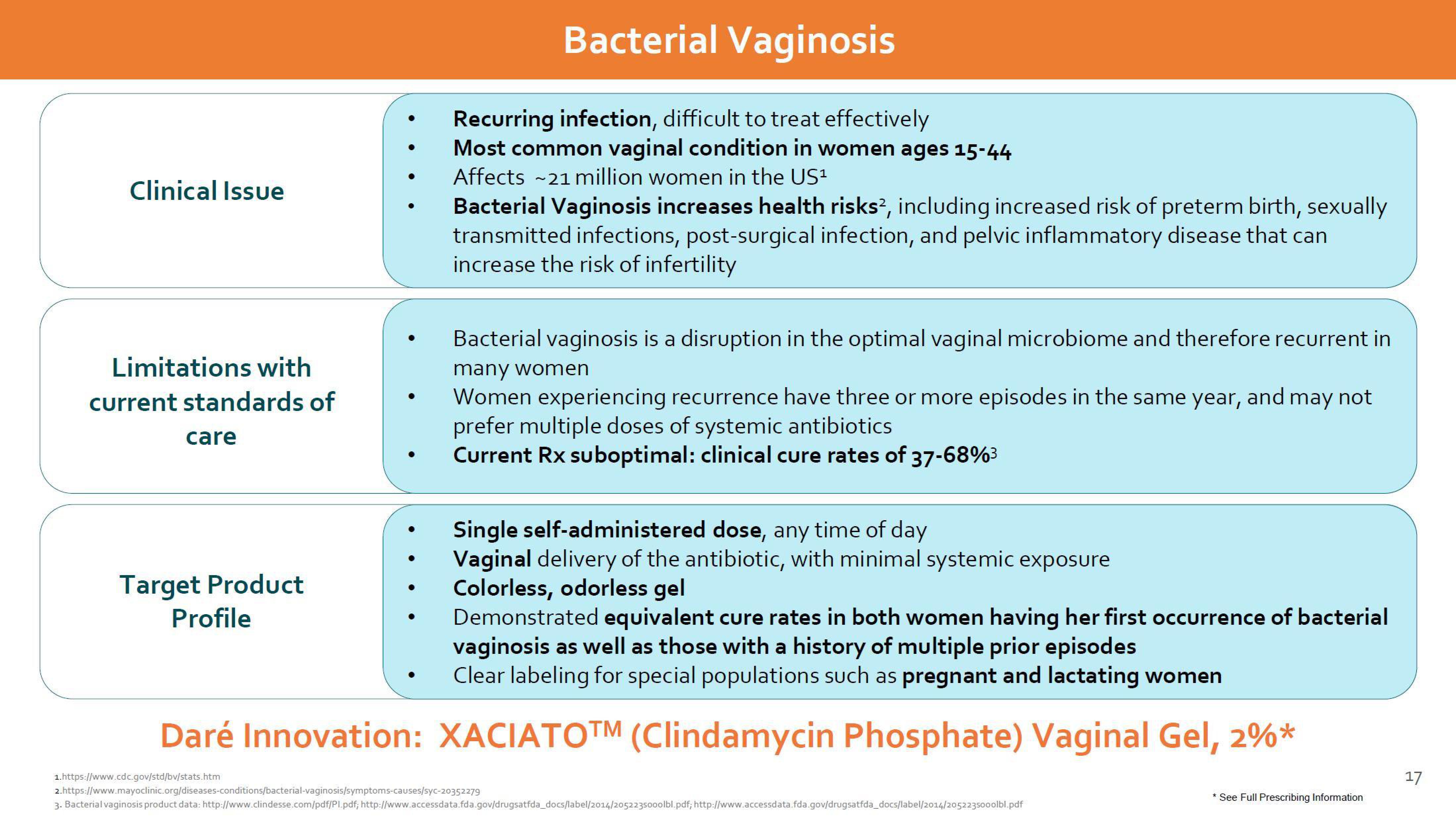
Clinical Issue
Limitations with
current standards of
care
Target Product
Profile
Bacterial Vaginosis
1.https://www.cdc.gov/std/bv/stats.htm
Recurring infection, difficult to treat effectively
Most common vaginal condition in women ages 15-44
Affects ~21 million women in the US¹
Bacterial Vaginosis increases health risks², including increased risk of preterm birth, sexually
transmitted infections, post-surgical infection, and pelvic inflammatory disease that can
increase the risk of infertility
Bacterial vaginosis is a disruption in the optimal vaginal microbiome and therefore recurrent in
many women
Women experiencing recurrence have three or more episodes in the same year, and may not
prefer multiple doses of systemic antibiotics
Current Rx suboptimal: clinical cure rates of 37-68%³
Single self-administered dose, any time of day
Vaginal delivery of the antibiotic, with minimal systemic exposure
Colorless, odorless gel
Demonstrated equivalent cure rates in both women having her first occurrence of bacterial
vaginosis as well as those with a history of multiple prior episodes
Clear labeling for special populations such as pregnant and lactating women
Daré Innovation: XACIATOTM (Clindamycin Phosphate) Vaginal Gel, 2%*
2.https://www.mayoclinic.org/diseases-conditions/bacterial-vaginosis/symptoms-causes/syc-20352279
3. Bacterial vaginosis product data: http://www.clindesse.com/pdf/Pl.pdf; http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/2052235000lbl.pdf; http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/2052235000lbl.pdf
* See Full Prescribing Information
17View entire presentation