AstraZeneca Results Presentation Deck
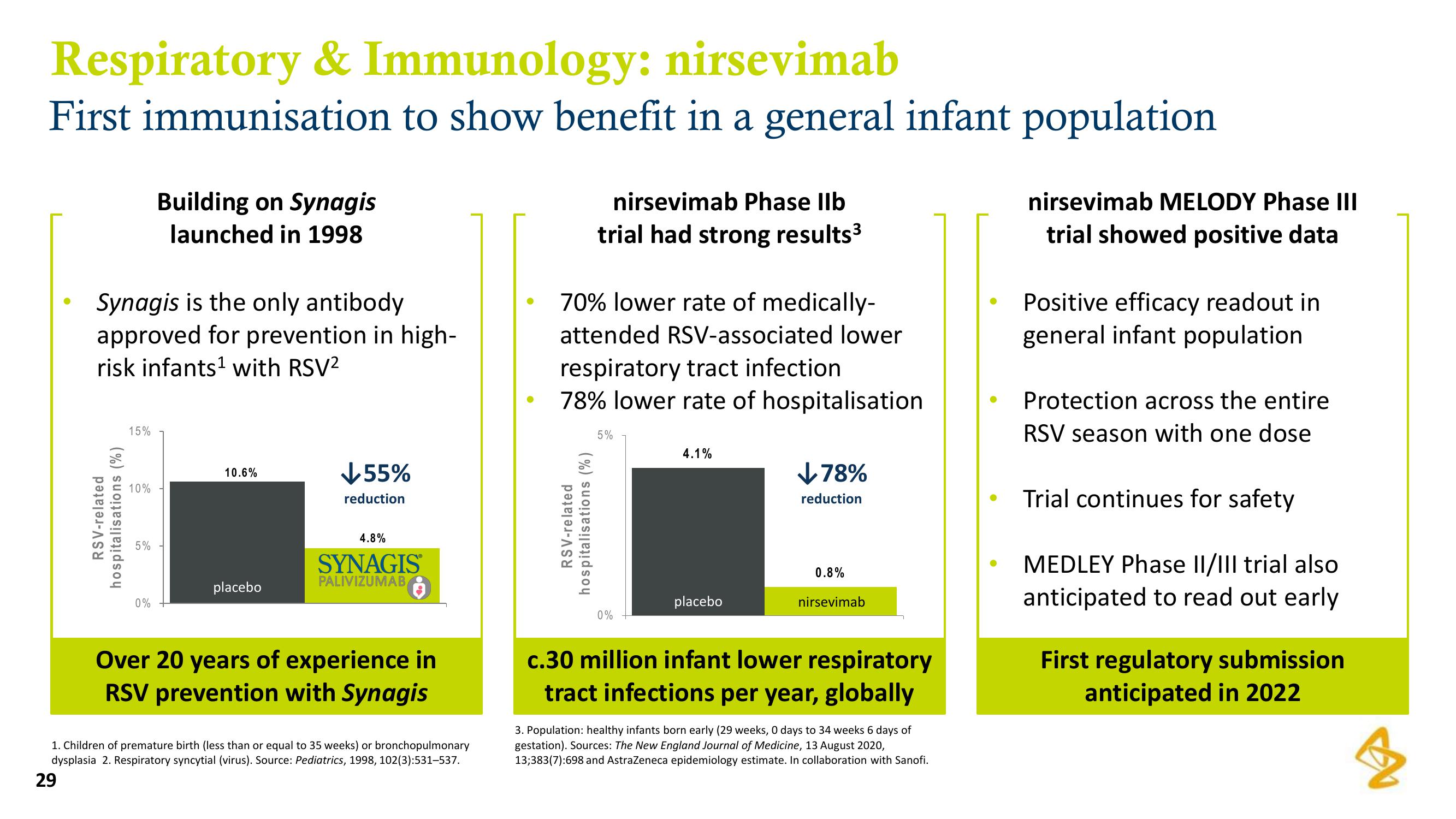
Respiratory & Immunology: nirsevimab
First immunisation to show benefit in a general infant population
Synagis is the only antibody
approved for prevention in high-
risk infants¹ with RSV²
RSV-related
hospitalisations (%)
15%
10%
5%
Building on Synagis
launched in 1998
0%
10.6%
placebo
↓55%
reduction
4.8%
SYNAGIS
8
PALIVIZUMAB
Over 20 years of experience in
RSV prevention with Synagis
1. Children of premature birth (less than or equal to 35 weeks) or bronchopulmonary
dysplasia 2. Respiratory syncytial (virus). Source: Pediatrics, 1998, 102(3):531-537.
29
nirsevimab Phase IIb
trial had strong results³
70% lower rate of medically-
attended RSV-associated lower
respiratory tract infection
78% lower rate of hospitalisation
RSV-related
hospitalisations (%)
5%
0%
4.1%
placebo
↓78%
reduction
0.8%
nirsevimab
c.30 million infant lower respiratory
tract infections per year, globally
3. Population: healthy infants born early (29 weeks, 0 days to 34 weeks 6 days of
gestation). Sources: The New England Journal of Medicine, 13 August 2020,
13;383(7):698 and AstraZeneca epidemiology estimate. In collaboration with Sanofi.
nirsevimab MELODY Phase III
trial showed positive data
Positive efficacy readout in
general infant population.
Protection across the entire
RSV season with one dose
Trial continues for safety
MEDLEY Phase II/III trial also
anticipated to read out early
First regulatory submission
anticipated in 2022
3View entire presentation