Ocuphire Pharma Investor Presentation Deck
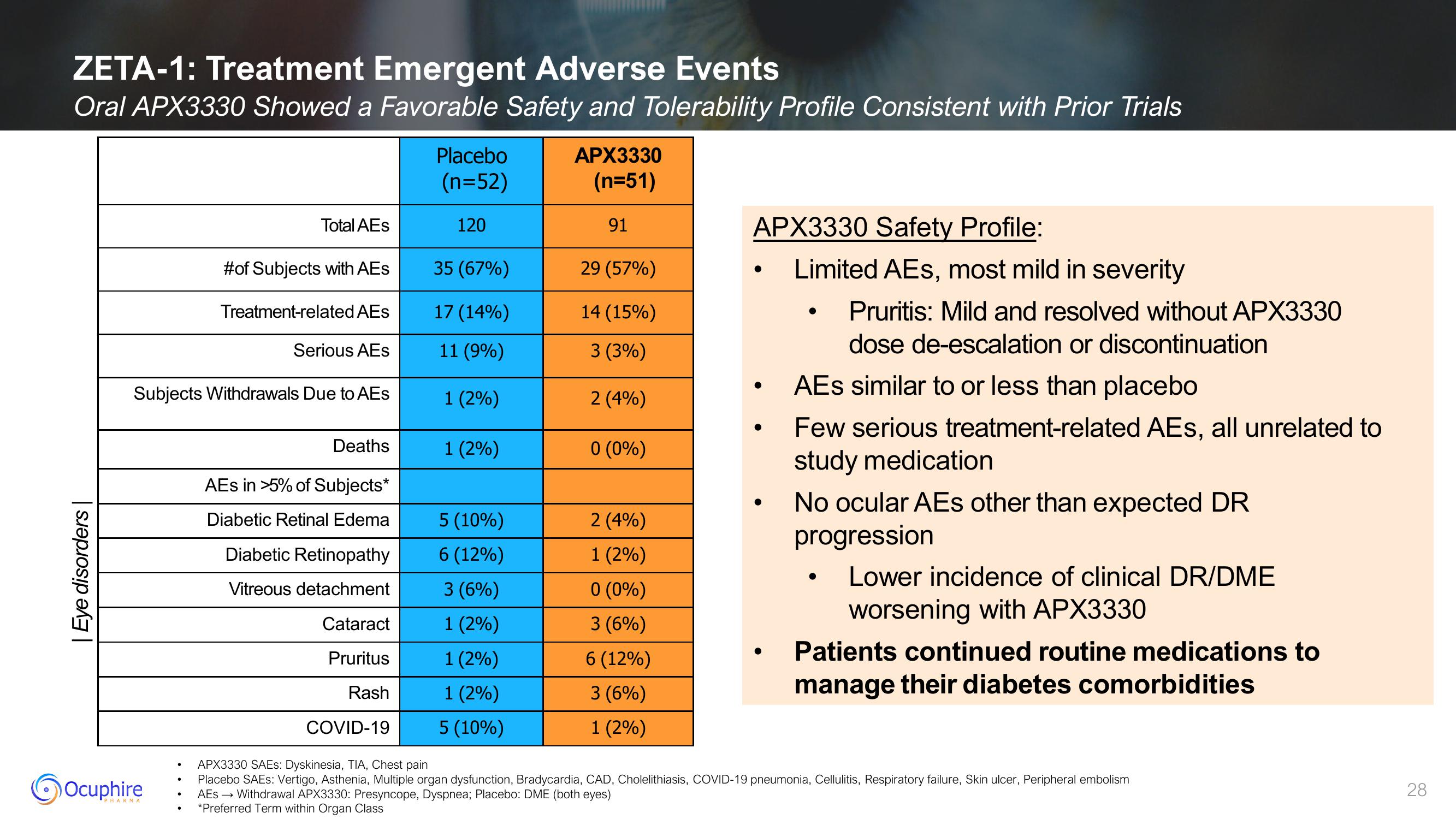
ZETA-1: Treatment Emergent Adverse Events
Oral APX3330 Showed a Favorable Safety and Tolerability Profile Consistent with Prior Trials
| Eye disorders |
Total AEs
Ocuphire
PHARMA
#of Subjects with AEs
Treatment-related AEs
Serious AEs
Subjects Withdrawals Due to AEs
Deaths
AES in >5% of Subjects*
Diabetic Retinal Edema
Diabetic Retinopathy
Vitreous detachment
Cataract
Pruritus
Rash
COVID-19
Placebo
(n=52)
120
35 (67%)
17 (14%)
11 (9%)
1 (2%)
1 (2%)
5 (10%)
6 (12%)
3 (6%)
1 (2%)
1 (2%)
1 (2%)
5 (10%)
APX3330
(n=51)
91
29 (57%)
14 (15%)
3 (3%)
2 (4%)
0 (0%)
2 (4%)
1 (2%)
0 (0%)
3 (6%)
6 (12%)
3 (6%)
1 (2%)
APX3330 Safety Profile:
Limited AEs, most mild in severity
●
Pruritis: Mild and resolved without APX3330
dose de-escalation or discontinuation
AEs similar to or less than placebo
Few serious treatment-related AEs, all unrelated to
study medication
No ocular AEs other than expected DR
progression
●
Lower incidence of clinical DR/DME
worsening with APX3330
Patients continued routine medications to
manage their diabetes comorbidities
APX3330 SAES: Dyskinesia, TIA, Chest pain
Placebo SAES: Vertigo, Asthenia, Multiple organ dysfunction, Bradycardia, CAD, Cholelithiasis, COVID-19 pneumonia, Cellulitis, Respiratory failure, Skin ulcer, Peripheral embolism
AES → Withdrawal APX3330: Presyncope, Dyspnea; Placebo: DME (both eyes)
*Preferred Term within Organ Class
28View entire presentation