Dare Bioscience Investor Presentation Deck
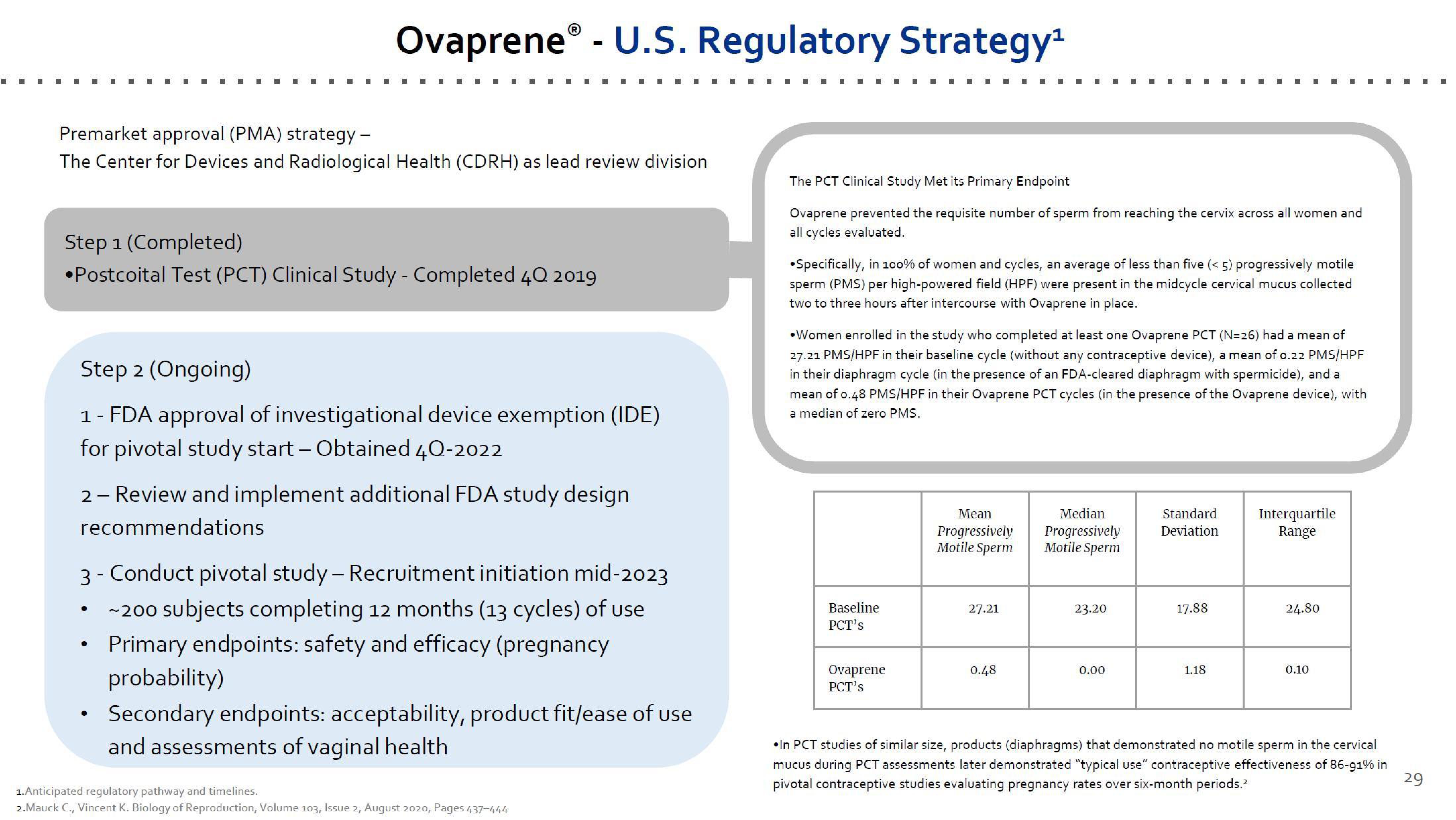
Premarket approval (PMA) strategy -
The Center for Devices and Radiological Health (CDRH) as lead review division
OvapreneⓇ - U.S. Regulatory Strategy¹
Step 1 (Completed)
•Postcoital Test (PCT) Clinical Study - Completed 4Q 2019
Step 2 (Ongoing)
1 - FDA approval of investigational device exemption (IDE)
for pivotal study start - Obtained 4Q-2022
2 - Review and implement additional FDA study design
recommendations
3- Conduct pivotal study - Recruitment initiation mid-2023
~200 subjects completing 12 months (13 cycles) of use
Primary endpoints: safety and efficacy (pregnancy
probability)
Secondary endpoints: acceptability, product fit/ease of use
and assessments of vaginal health
●
●
1. Anticipated regulatory pathway and timelines.
2.Mauck C., Vincent K. Biology of Reproduction, Volume 103, Issue 2, August 2020, Pages 437-444
The PCT Clinical Study Met its Primary Endpoint
Ovaprene prevented the requisite number of sperm from reaching the cervix across all women and
all cycles evaluated.
Specifically, in 100% of women and cycles, an average of less than five (<5) progressively motile
sperm (PMS) per high-powered field (HPF) were present in the midcycle cervical mucus collected
two to three hours after intercourse with Ovaprene in place.
•Women enrolled in the study who completed at least one Ovaprene PCT (N=26) had a mean of
27.21 PMS/HPF in their baseline cycle (without any contraceptive device), a mean of 0.22 PMS/HPF
in their diaphragm cycle (in the presence of an FDA-cleared diaphragm with spermicide), and a
mean of 0.48 PMS/HPF in their Ovaprene PCT cycles (in the presence of the Ovaprene device), with
a median of zero PMS.
Baseline
PCT's
Ovaprene
PCT's
Mean
Progressively
Motile Sperm
27.21
0.48
Median
Progressively
Motile Sperm
23.20
0.00
Standard
Deviation
17.88
1.18
Interquartile
Range
24.80
0.10
•In PCT studies of similar size, products (diaphragms) that demonstrated no motile sperm in the cervical
mucus during PCT assessments later demonstrated "typical use" contraceptive effectiveness of 86-91% in
pivotal contraceptive studies evaluating pregnancy rates over six-month periods.²
29View entire presentation