BioNTech Results Presentation Deck
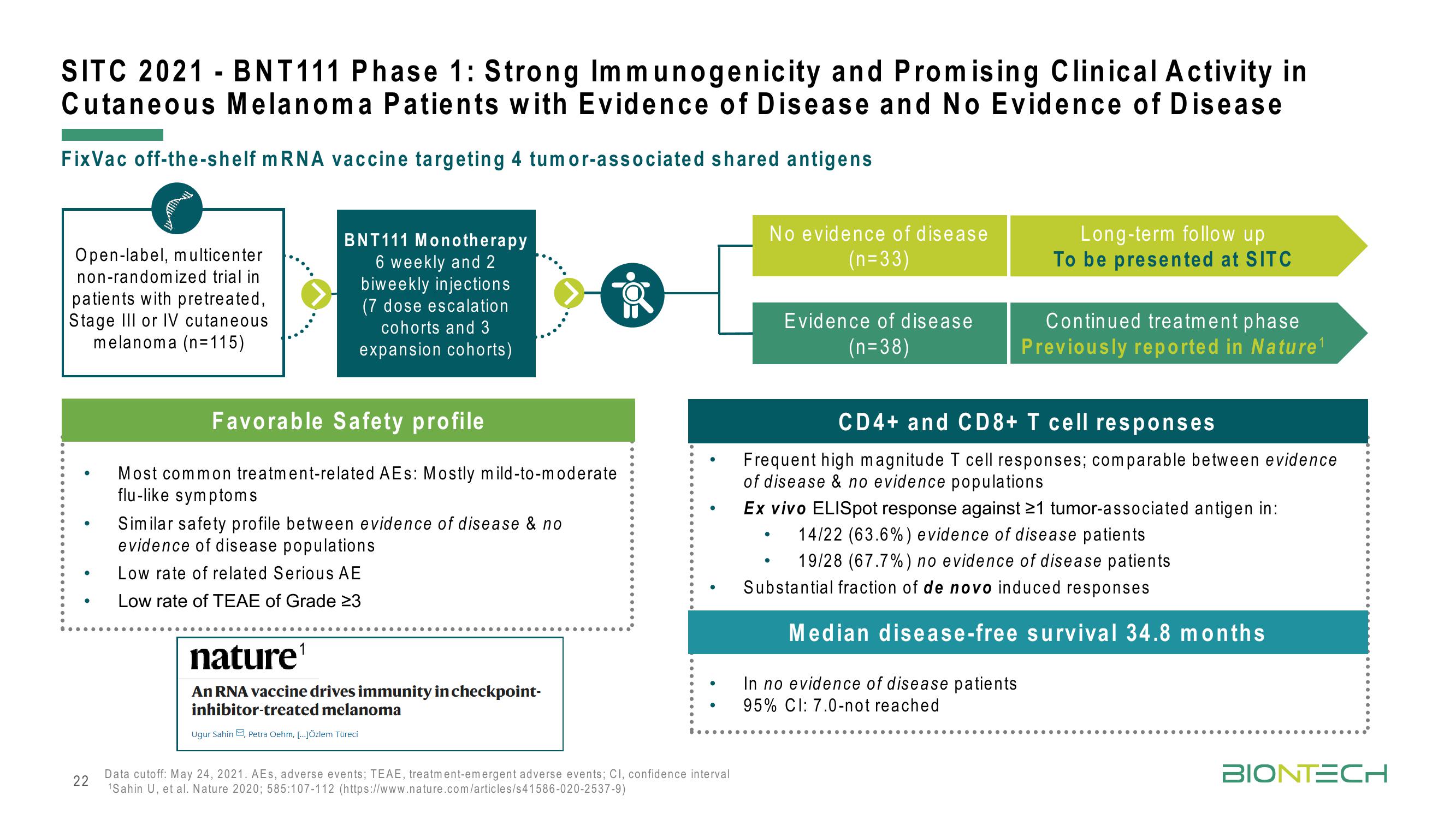
SITC 2021 - BNT111 Phase 1: Strong Immunogenicity and Promising Clinical Activity in
Cutaneous Melanoma Patients with Evidence of Disease and No Evidence of Disease
FixVac off-the-shelf mRNA vaccine targeting 4 tumor-associated shared antigens
Open-label, multicenter
non-randomized trial in
patients with pretreated,
Stage III or IV cutaneous
melanoma (n=115)
●
●
22
BNT111 Monotherapy
6 weekly and 2
biweekly injections
(7 dose escalation
cohorts and 3
expansion cohorts)
Favorable Safety profile
Most common treatment-related AEs: Mostly mild-to-moderate
flu-like symptoms
Similar safety profile between evidence of disease & no
evidence of disease populations
Low rate of related Serious AE
Low rate of TEAE of Grade 23
nature¹
An RNA vaccine drives immunity in checkpoint-
inhibitor-treated melanoma
Ugur Sahin, Petra Oehm, [...]Özlem Türeci
•O
Data cutoff: May 24, 2021. AEs, adverse events; TEAE, treatment-emergent adverse events; CI, confidence interval
¹Sahin U, et al. Nature 2020; 585:107-112 (https://www.nature.com/articles/s41586-020-2537-9)
No evidence of disease
(n=33)
Evidence of disease
(n=38)
Long-term follow up
To be presented at SITC
Continued treatment phase
Previously reported in Nature ¹
CD4+ and CD8+ T cell responses
Frequent high magnitude T cell responses; comparable between evidence
of disease & no evidence populations
In no evidence of disease patients
95% CI: 7.0-not reached
Ex vivo ELISpot response against ≥1 tumor-associated antigen in:
14/22 (63.6%) evidence of disease patients
19/28 (67.7%) no evidence of disease patients
Substantial fraction of de novo induced responses
Median disease-free survival 34.8 months
BIONTECHView entire presentation