Legend Biotech Results Presentation Deck
9
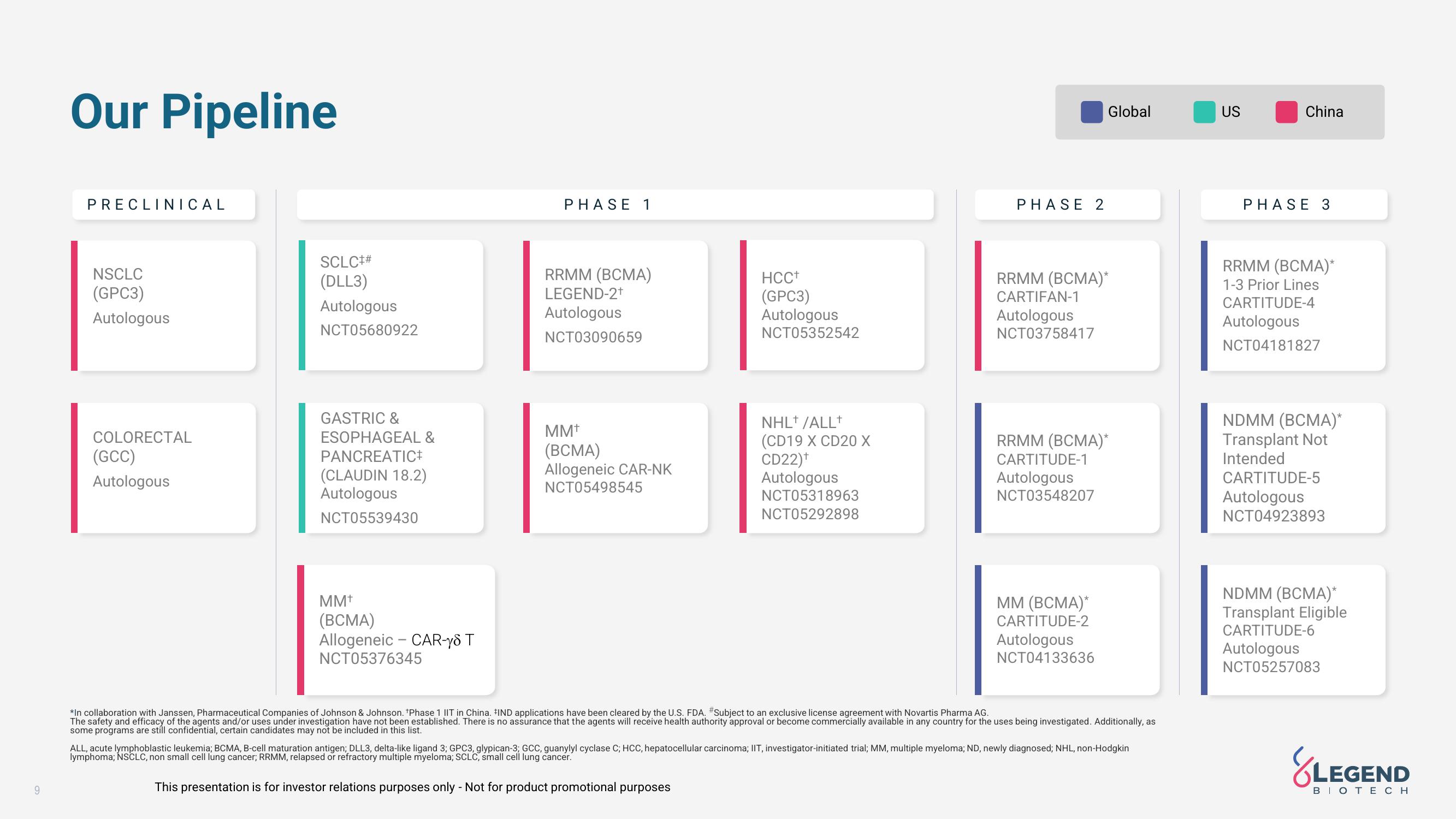
Our Pipeline
PRECLINICAL
NSCLC
(GPC3)
Autologous
COLORECTAL
(GCC)
Autologous
SCLC##
(DLL3)
Autologous
NCT05680922
GASTRIC &
ESOPHAGEAL &
PANCREATIC+
(CLAUDIN 18.2)
Autologous
NCT05539430
MMT
(BCMA)
Allogeneic - CAR-18 T
NCT05376345
PHASE 1
RRMM (BCMA)
LEGEND-2+
Autologous
NCT03090659
MMT
(BCMA)
Allogeneic CAR-NK
NCT05498545
HCC+
(GPC3)
Autologous
NCT05352542
NHL+/ALL+
(CD19 X CD20 X
CD22)+
Autologous
NCT05318963
NCT05292898
PHASE 2
Global
RRMM (BCMA)*
CARTIFAN-1
Autologous
NCT03758417
RRMM (BCMA)*
CARTITUDE-1
Autologous
NCT03548207
MM (BCMA)*
CARTITUDE-2
Autologous
NCT04133636
*In collaboration with Janssen, Pharmaceutical Companies of Johnson & Johnson. *Phase 1 IIT in China. #IND applications have been cleared by the U.S. FDA. #Subject to an exclusive license agreement with Novartis Pharma AG.
The safety and efficacy of the agents and/or uses under investigation have not been established. There is no assurance that the agents will receive health authority approval or become commercially available in any country for the uses being investigated. Additionally, as
some programs are still confidential, certain candidates may not be included in this list.
ALL, acute lymphoblastic leukemia; BCMA, B-cell maturation antigen; DLL3, delta-like ligand 3; GPC3, glypican-3; GCC, guanylyl cyclase C; HCC, hepatocellular carcinoma; IIT, investigator-initiated trial; MM, multiple myeloma; ND, newly diagnosed; NHL, non-Hodgkin
lymphoma; NSCLC, non small cell lung cancer; RRMM, relapsed or refractory multiple myeloma; SCLC, small cell lung cancer.
This presentation is for investor relations purposes only - Not for product promotional purposes
US
China
PHASE 3
RRMM (BCMA)*
1-3 Prior Lines
CARTITUDE-4
Autologous
NCT04181827
NDMM (BCMA)*
Transplant Not
Intended
CARTITUDE-5
Autologous
NCT04923893
NDMM (BCMA)*
Transplant Eligible
CARTITUDE-6
Autologous
NCT05257083
LEGEND
BIOTECHView entire presentation