Bausch+Lomb Results Presentation Deck
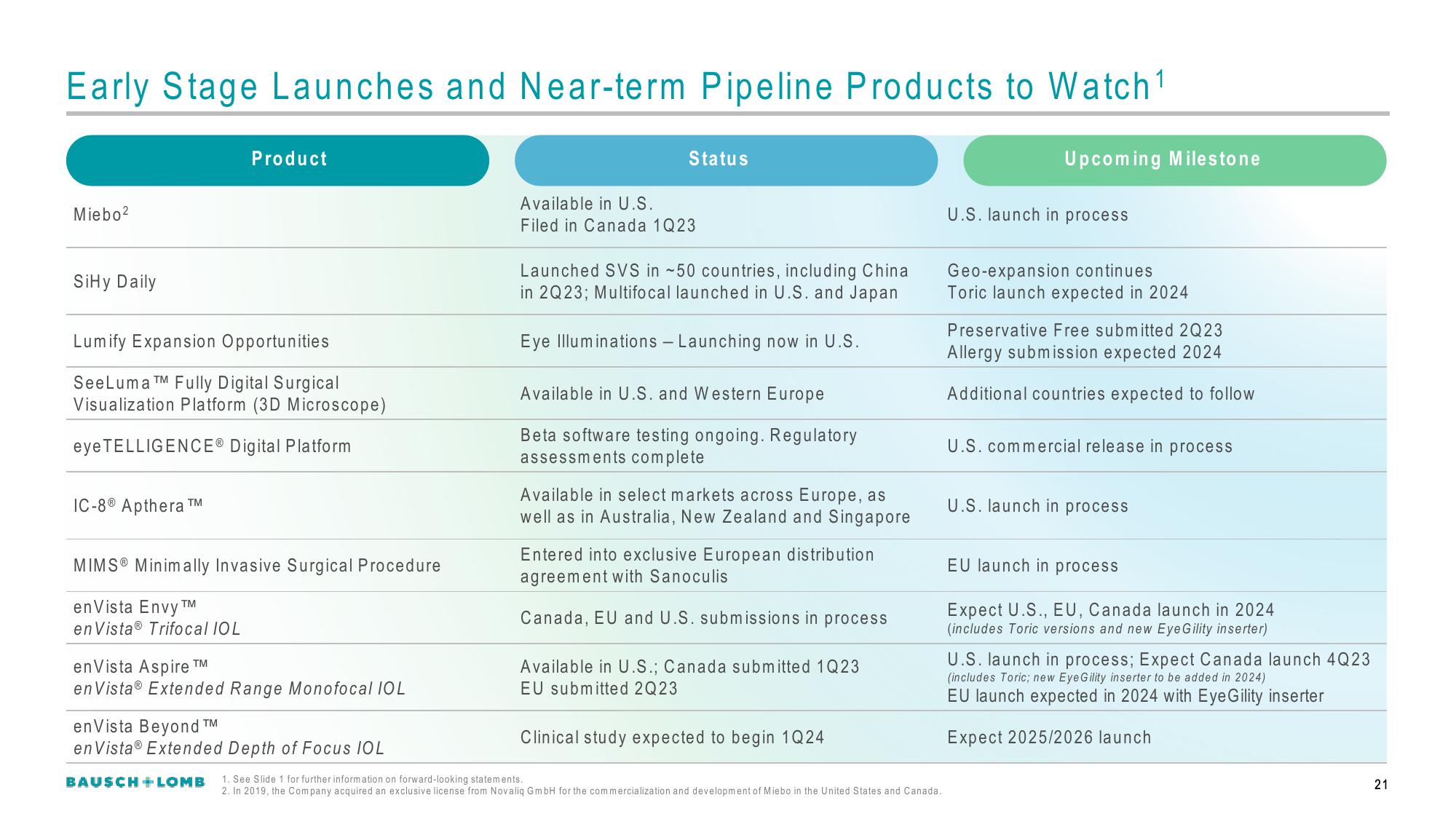
Early Stage Launches and Near-term Pipeline Products to Watch ¹
Miebo²
SiHy Daily
Product
Lumify Expansion Opportunities
SeeLuma TM Fully Digital Surgical
Visualization Platform (3D Microscope)
eye TELLIGENCE® Digital Platform
IC-8 Apthera ™
MIMSⓇ Minimally Invasive Surgical Procedure
en Vista Envy ™M
en Vista® Trifocal IOL
en Vista Aspire ™
en Vista® Extended Range Monofocal IOL
en Vista Beyond ™
en Vista® Extended Depth of Focus IOL
BAUSCH+ LOMB
Status
Available in U.S.
Filed in Canada 1Q23
Launched SVS in -50 countries, including China
in 2Q23; Multifocal launched in U.S. and Japan
Eye Illuminations - Launching now in U.S.
Available in U.S. and Western Europe
Beta software testing ongoing. Regulatory
assessments complete
Available in select markets across Europe, as
well as in Australia, New Zealand and Singapore
Entered into exclusive European distribution
agreement with Sanoculis
Canada, EU and U.S. submissions in process
Available in U.S.; Canada submitted 1Q23
EU submitted 2Q23
Clinical study expected to begin 1Q24
1. See Slide 1 for further information on forward-looking statements.
2. In 2019, the Company acquired an exclusive license from Novaliq GmbH for the commercialization and development of Miebo in the United States and Canada.
Upcoming Milestone
U.S. launch in process
Geo-expansion continues
Toric launch expected in 2024
Preservative Free submitted 2Q23
Allergy submission expected 2024
Additional countries expected to follow
U.S. commercial release in process
U.S. launch in process
EU launch in process
Expect U.S., EU, Canada launch in 2024
(includes Toric versions and new EyeGility inserter)
U.S. launch in process; Expect Canada launch 4Q23
(includes Toric; new EyeGility inserter to be added in 2024)
EU launch expected in 2024 with EyeGility inserter
Expect 2025/2026 launch
21View entire presentation