Context Therapeutics Investor Presentation Deck
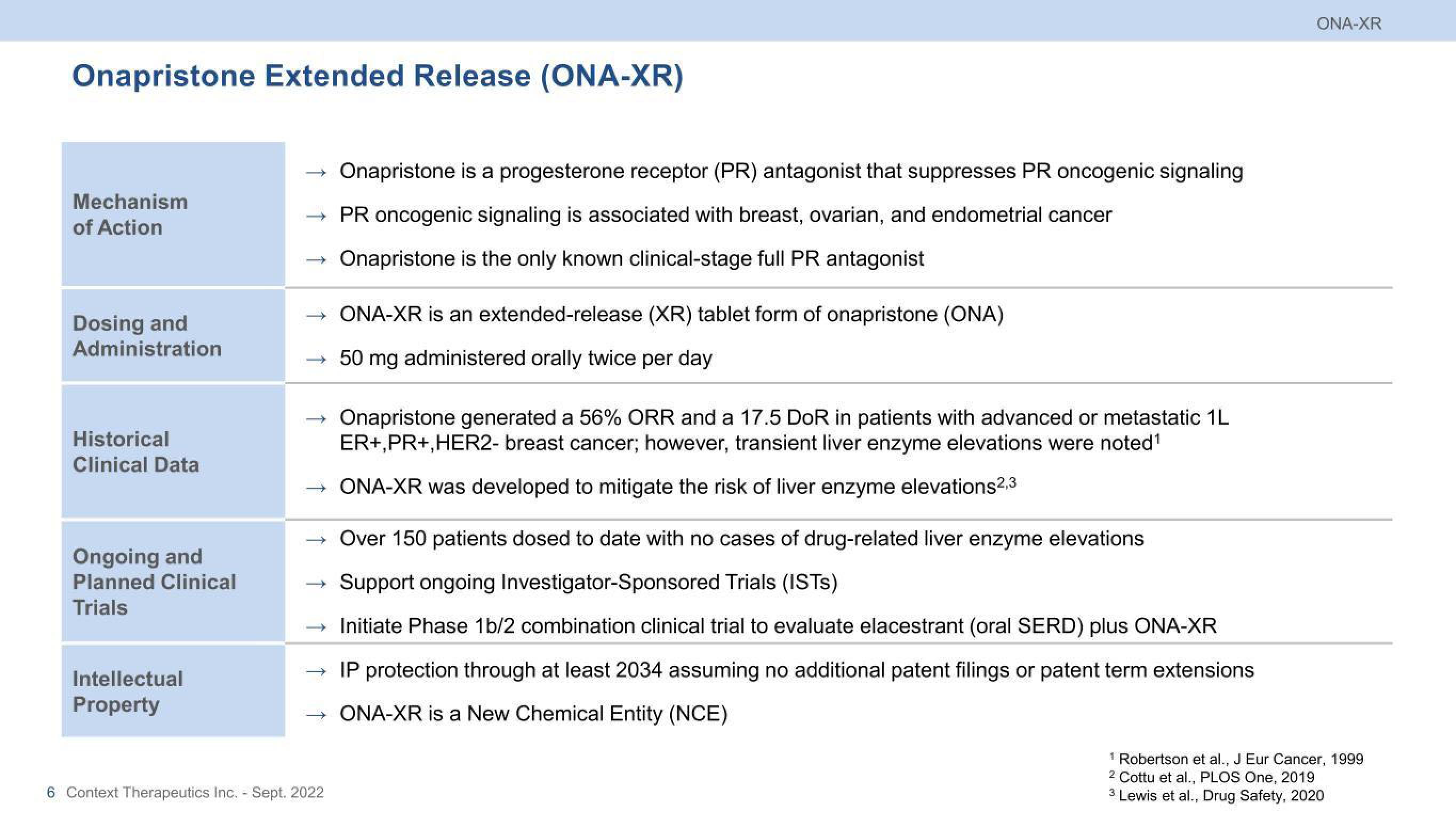
Onapristone Extended Release (ONA-XR)
Mechanism
of Action
Dosing and
Administration
Historical
Clinical Data
Ongoing and
Planned Clinical
Trials
Intellectual
Property
6 Context Therapeutics Inc. - Sept. 2022
Onapristone is a progesterone receptor (PR) antagonist that suppresses PR oncogenic signaling
PR oncogenic signaling is associated with breast, ovarian, and endometrial cancer
Onapristone is the only known clinical-stage full PR antagonist
ONA-XR is an extended-release (XR) tablet form of onapristone (ONA)
50 mg administered orally twice per day
Onapristone generated a 56% ORR and a 17.5 DoR in patients with advanced or metastatic 1L
ER+,PR+, HER2- breast cancer; however, transient liver enzyme elevations were noted¹
ONA-XR was developed to mitigate the risk of liver enzyme elevations2,3
Over 150 patients dosed to date with no cases of drug-related liver enzyme elevations
Support ongoing Investigator-Sponsored Trials (ISTs)
Initiate Phase 1b/2 combination clinical trial to evaluate elacestrant (oral SERD) plus ONA-XR
IP protection through at least 2034 assuming no additional patent filings or patent term extensions
ONA-XR is a New Chemical Entity (NCE)
ONA-XR
¹ Robertson et al., J Eur Cancer, 1999
2 Cottu et al., PLOS One, 2019
3 Lewis et al., Drug Safety, 2020View entire presentation