IDEAYA Biosciences Interim IDE397 Phase 1 Clinical Data and Q1 2022 Corporate Update
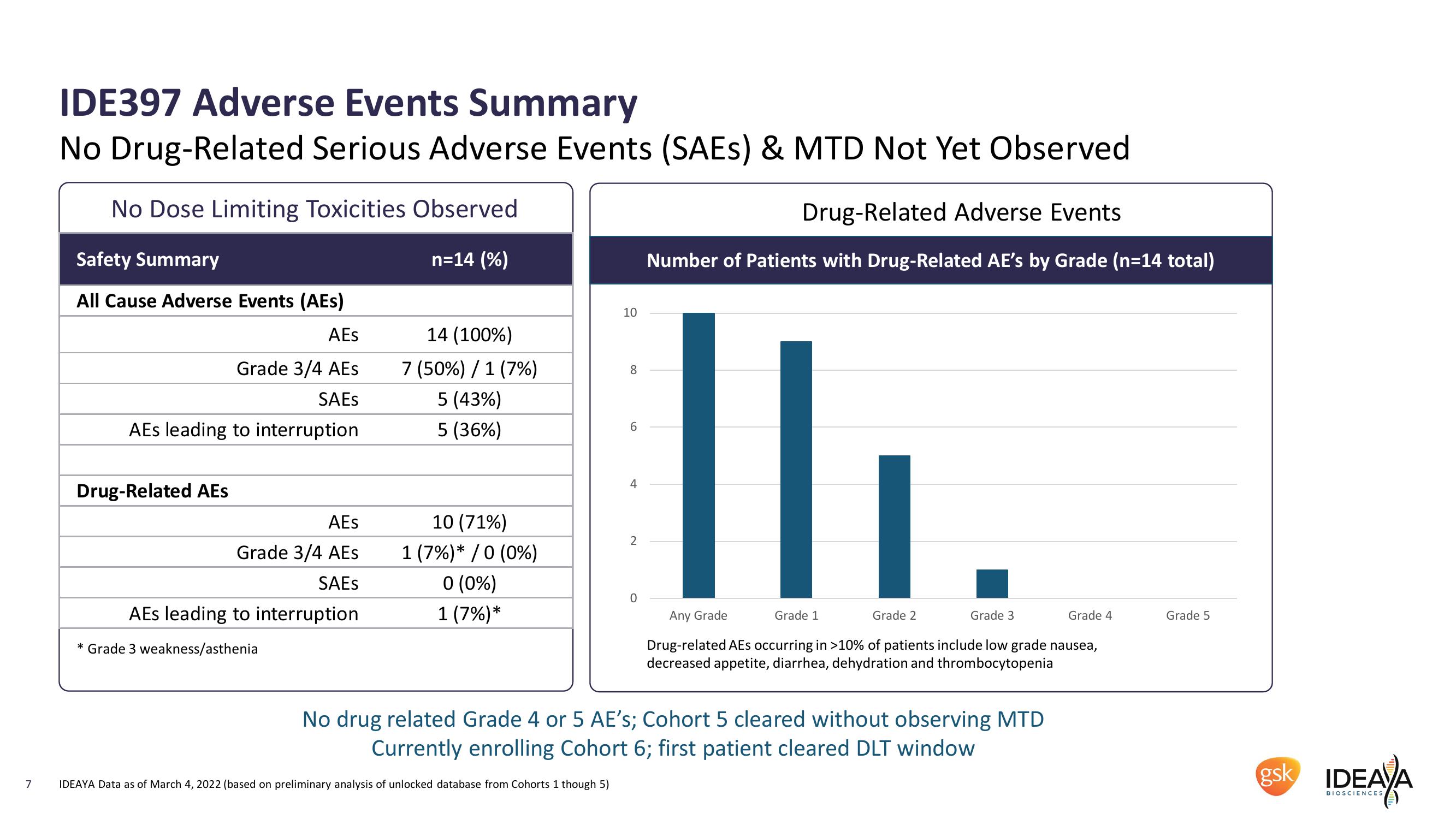
IDE397 Adverse Events Summary
No Drug-Related Serious Adverse Events (SAEs) & MTD Not Yet Observed
No Dose Limiting Toxicities Observed
Drug-Related Adverse Events
Number of Patients with Drug-Related AE's by Grade (n=14 total)
Safety Summary
n=14 (%)
All Cause Adverse Events (AEs)
10
AEs
14 (100%)
Grade 3/4 AES
SAES
7 (50%) / 1 (7%)
8
5 (43%)
AEs leading to interruption
Drug-Related AEs
5 (36%)
AEs
10 (71%)
2
Grade 3/4 AEs
SAES
1 (7%)* / 0 (0%)
AEs leading to interruption
0 (0%)
1 (7%)*
* Grade 3 weakness/asthenia
6
4
III.
0
Any Grade
Grade 1
Grade 2
Grade 3
Grade 4
Grade 5
Drug-related AEs occurring in >10% of patients include low grade nausea,
decreased appetite, diarrhea, dehydration and thrombocytopenia
No drug related Grade 4 or 5 AE's; Cohort 5 cleared without observing MTD
Currently enrolling Cohort 6; first patient cleared DLT window
7
IDEAYA Data as of March 4, 2022 (based on preliminary analysis of unlocked database from Cohorts 1 though 5)
gsk IDEAA
IDEAYA
BIOSCIENCESView entire presentation