Imara M&A
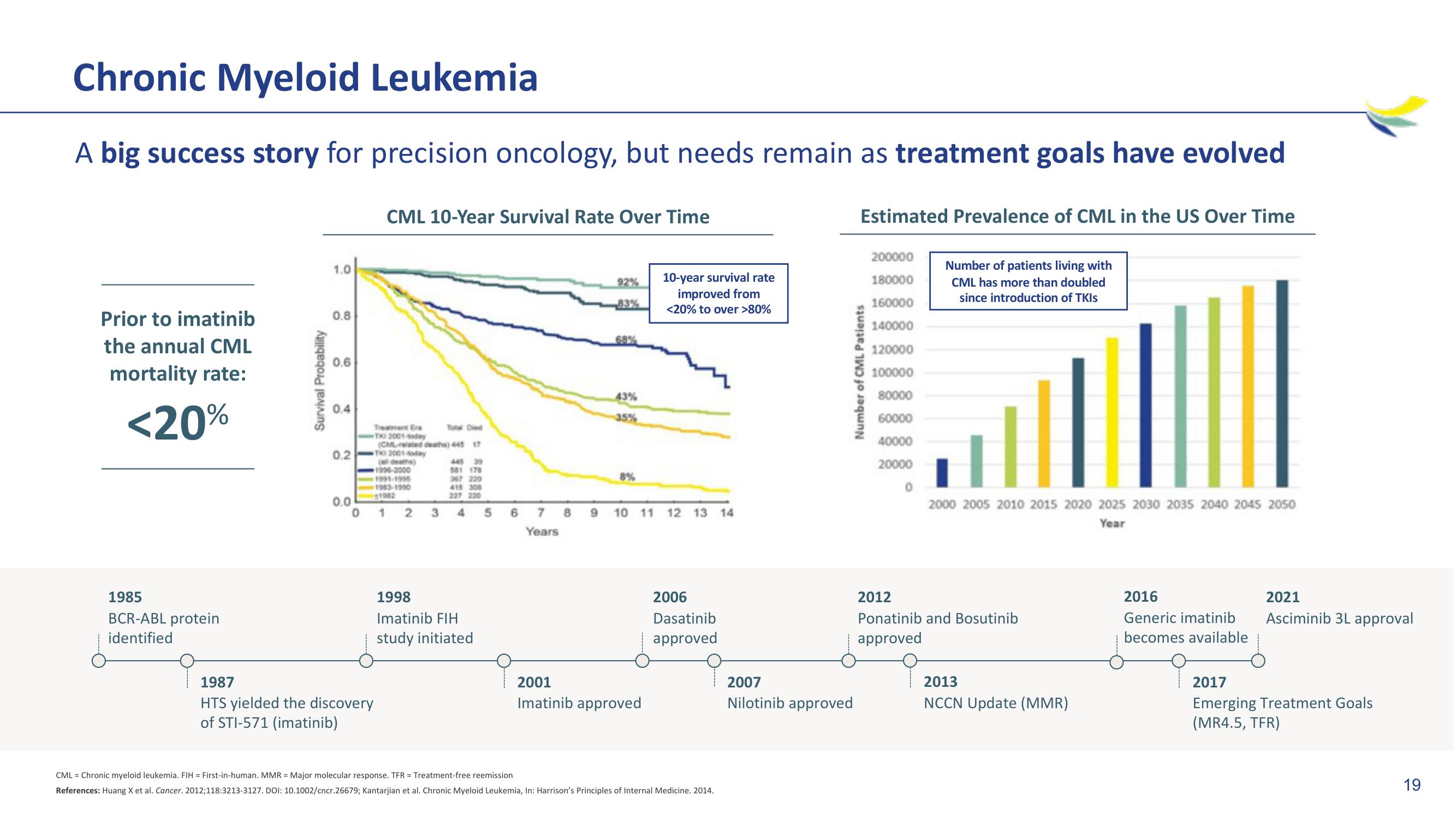
Chronic Myeloid Leukemia
A big success story for precision oncology, but needs remain as treatment goals have evolved
Prior to imatinib
the annual CML
mortality rate:
<20%
1985
BCR-ABL protein
identified
Survival Probability
1.0
0.8
0.6
0.4
0.2
0.0
0
1987
HTS yielded the discovery
of STI-571 (imatinib)
CML 10-Year Survival Rate Over Time
TK 2001-day
(CML-related deaths) 445 17
Ti 2001-40day
(all deaths)
1996-2000
581 178
367 220
415 308
227 220
1983-1990
1982
1 2 3 4
1998
Imatinib FIH
study initiated
5 6
7
Years
8
9
92%
83%
43%
35%
8%
10 11
2001
Imatinib approved
10-year survival rate
improved from
<20% to over >80%
12 13 14
2006
Dasatinib
approved
CML = Chronic myeloid leukemia. FIH = First-in-human. MMR = Major molecular response. TFR = Treatment-free reemission
References: Huang X et al. Cancer. 2012;118:3213-3127. DOI: 10.1002/cncr.26679; Kantarjian et al. Chronic Myeloid Leukemia, In: Harrison's Principles of Internal Medicine. 2014.
2007
Nilotinib approved
Estimated Prevalence of CML in the US Over Time
Number of CML Patients
200000
180000
160000
140000
120000
100000
80000
60000
40000
20000
Number of patients living with
CML has more than doubled
since introduction of TKIs
2000 2005 2010 2015 2020 2025 2030 2035 2040 2045 2050
Year
2012
Ponatinib and Bosutinib
approved
2013
NCCN Update (MMR)
2016
Generic imatinib
becomes available
2021
Asciminib 3L approval
2017
Emerging Treatment Goals
(MR4.5, TFR)
19View entire presentation