BioNTech Investor Day Presentation Deck
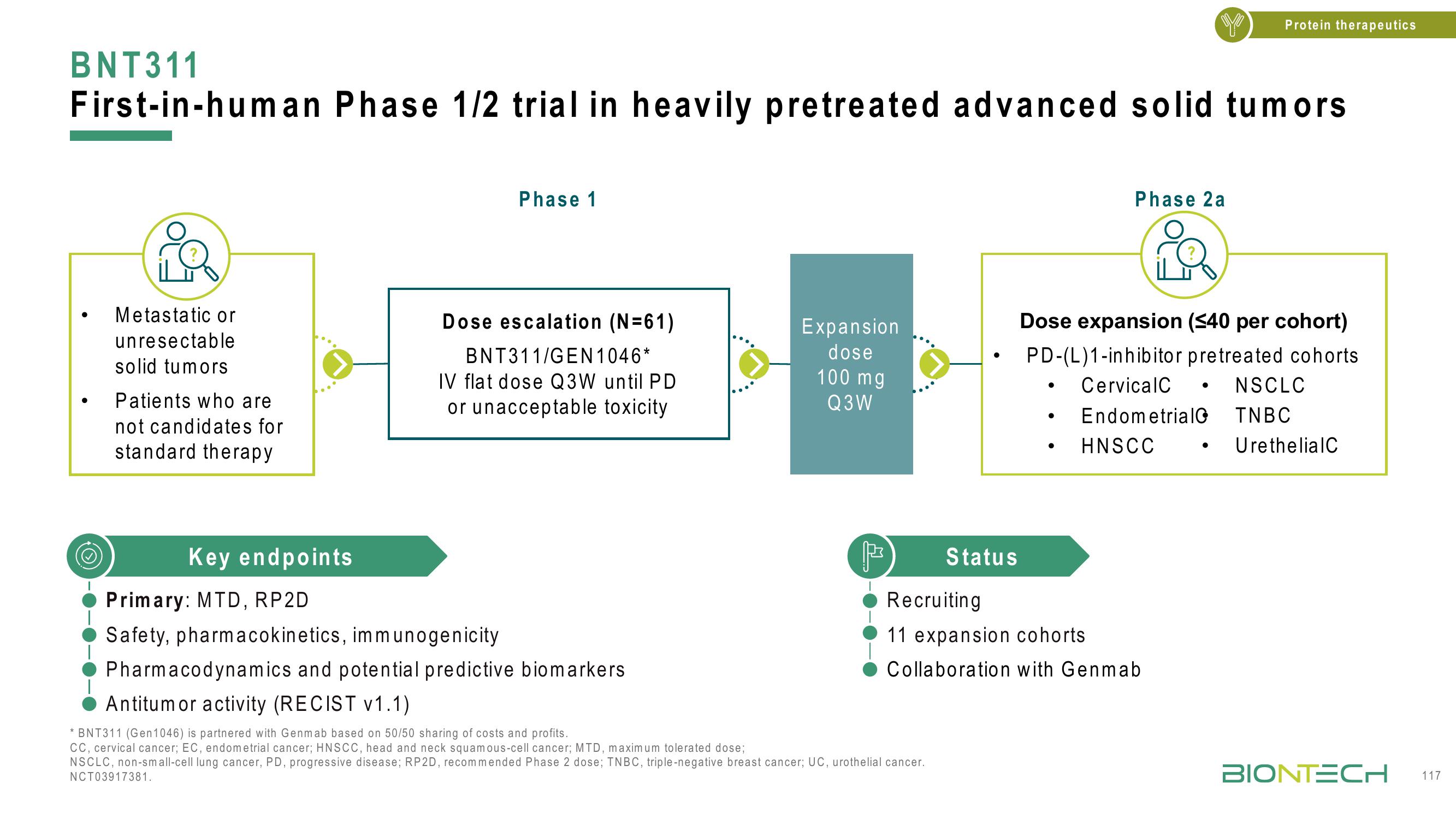
Metastatic or
unresectable
solid tumors
Patients who are
not candidates for
standard therapy
BNT311
First-in-human Phase 1/2 trial in heavily pretreated advanced solid tumors
Key endpoints
Phase 1
Dose escalation (N=61)
BNT311/GEN1046*
IV flat dose Q3W until PD
or unacceptable toxicity
Primary: MTD, RP2D
Safety, pharmacokinetics, immunogenicity
Expansion
dose
100 mg
Q3W
—
-
14
Status
Pharmacodynamics and potential predictive biomarkers
Antitumor activity (RECIST v1.1)
* BNT311 (Gen1046) is partnered with Genmab based on 50/50 sharing of costs and profits.
CC, cervical cancer; EC, endometrial cancer; HNSCC, head and neck squamous-cell cancer; MTD, maximum tolerated dose;
NSCLC, non-small-cell lung cancer, PD, progressive disease; RP2D, recommended Phase 2 dose; TNBC, triple-negative breast cancer; UC, urothelial cancer.
NCT03917381.
●
●
Phase 2a
Dose expansion (≤40 per cohort)
PD-(L)1-inhibitor pretreated cohorts.
Cervical C
Endometrial
HNSCC
Recruiting
11 expansion cohorts
Collaboration with Genmab
Y
●
Protein therapeutics
●
NSCLC
TNBC
UrethelialC
BIONTECH
117View entire presentation