Kymera Results Presentation Deck
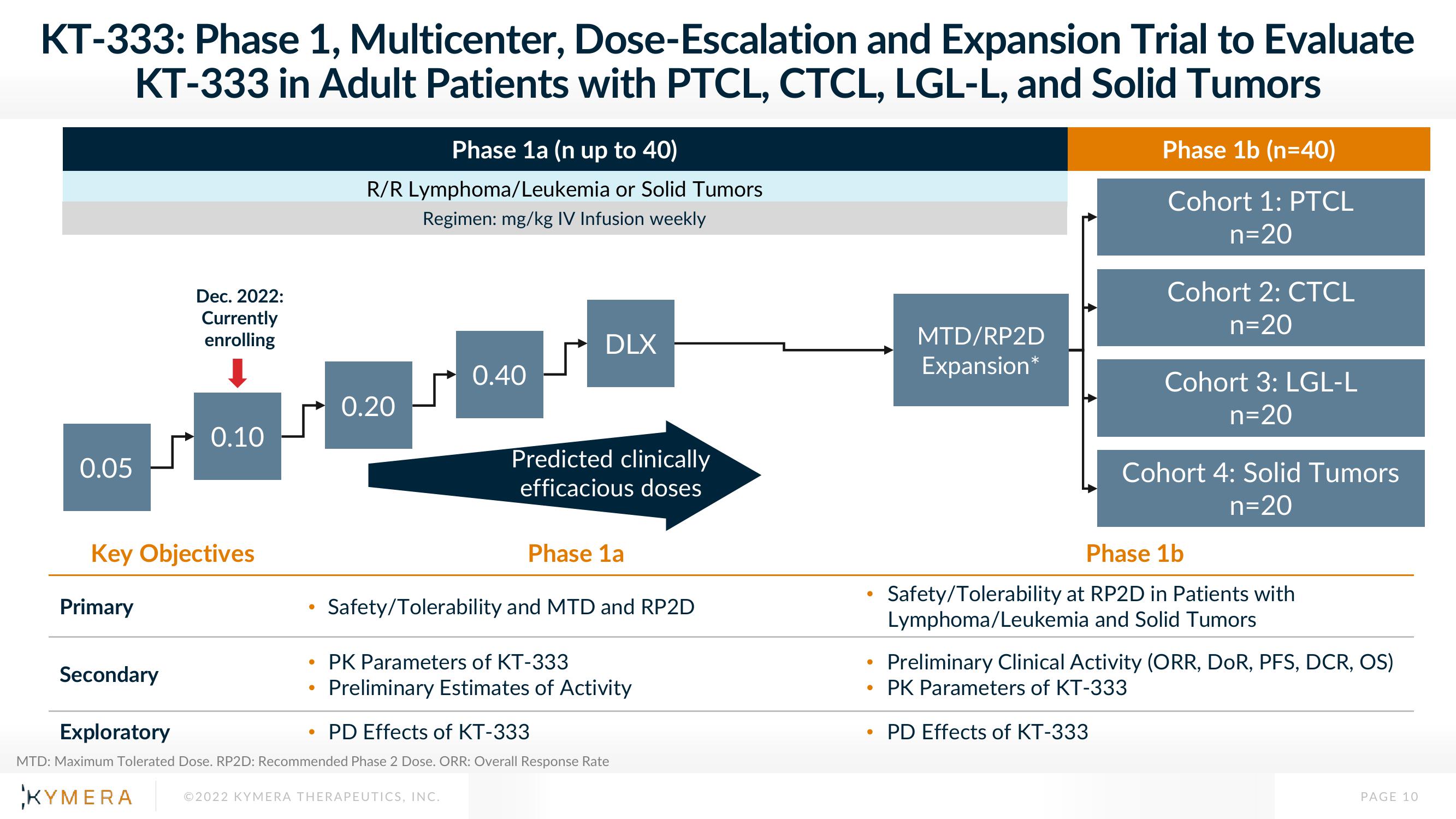
KT-333: Phase 1, Multicenter, Dose-Escalation and Expansion Trial to Evaluate
KT-333 in Adult Patients with PTCL, CTCL, LGL-L, and Solid Tumors
0.05
Primary
Dec. 2022:
Currently
enrolling
Key Objectives
Secondary
0.10
●
●
●
Phase 1a (n up to 40)
R/R Lymphoma/Leukemia or Solid Tumors
Regimen: mg/kg IV Infusion weekly
0.20
0.40
DLX
Predicted clinically
efficacious doses
Phase 1a
Safety/Tolerability and MTD and RP2D
PK Parameters of KT-333
Preliminary Estimates of Activity
Exploratory
PD Effects of KT-333
MTD: Maximum Tolerated Dose. RP2D: Recommended Phase 2 Dose. ORR: Overall Response Rate
KYMERA ©2022 KYMERA THERAPEUTICS, INC.
●
●
MTD/RP2D
Expansion*
●
Phase 1b (n=40)
Cohort 1: PTCL
n=20
Phase 1b
• Safety/Tolerability at RP2D in Patients with
Lymphoma/Leukemia and Solid Tumors
Cohort 2: CTCL
n=20
PD Effects of KT-333
Cohort 3: LGL-L
n=20
Cohort 4: Solid Tumors
n=20
Preliminary Clinical Activity (ORR, DOR, PFS, DCR, OS)
PK Parameters of KT-333
PAGE 10View entire presentation