Ocuphire Pharma Results Presentation Deck
RM
9
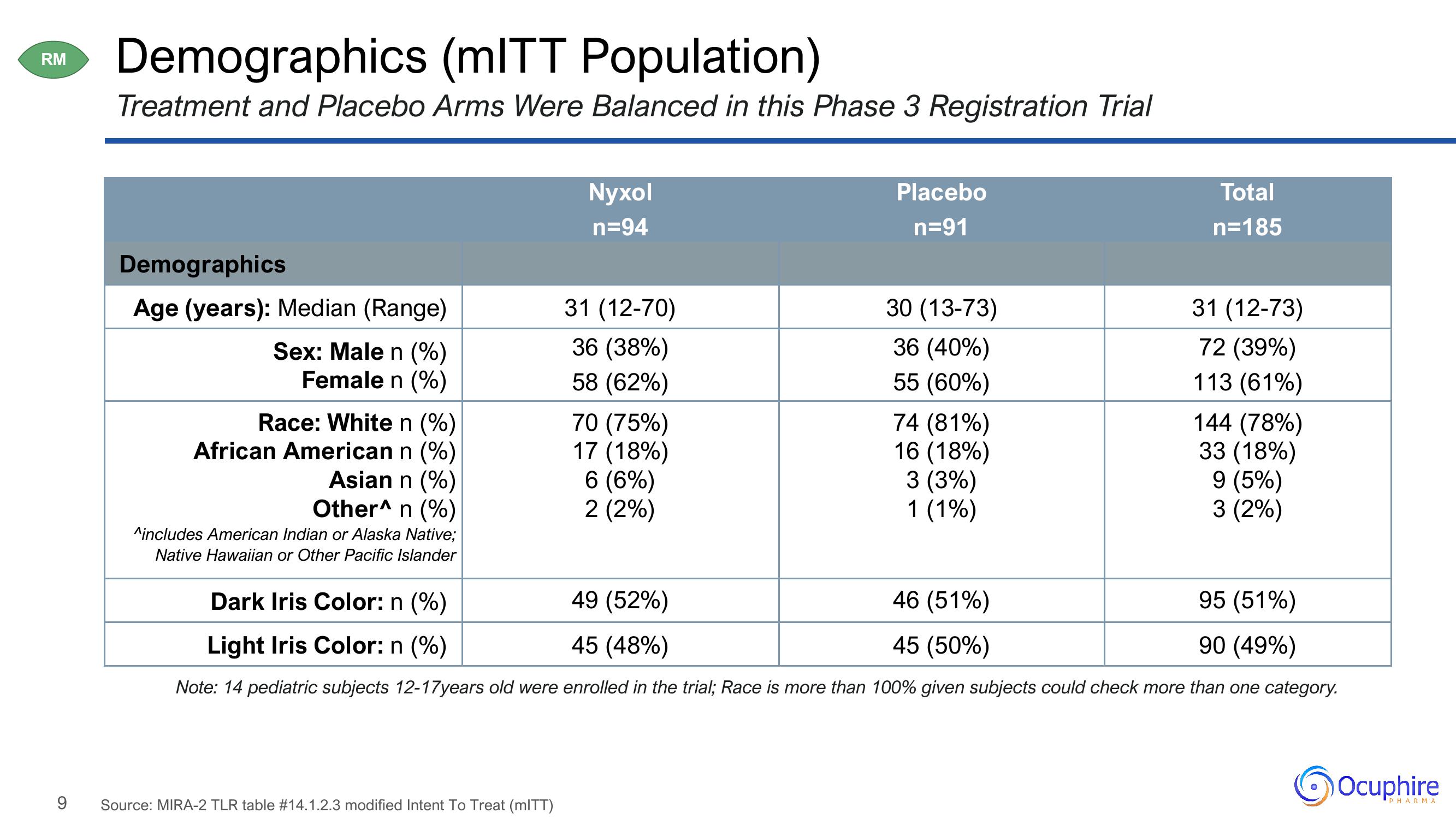
Demographics (mITT Population)
Treatment and Placebo Arms Were Balanced in this Phase 3 Registration Trial
Demographics
Age (years): Median (Range)
Sex: Male n (%)
Female n (%)
Race: White n (%)
African American n (%)
Asian n (%)
Other^ n (%)
^includes American Indian or Alaska Native;
Native Hawaiian or Other Pacific Islander
Nyxol
n=94
Source: MIRA-2 TLR table #14.1.2.3 modified Intent To Treat (mITT)
31 (12-70)
36 (38%)
58 (62%)
70 (75%)
17 (18%)
6 (6%)
2 (2%)
Placebo
n=91
30 (13-73)
36 (40%)
55 (60%)
74 (81%)
16 (18%)
3 (3%)
1 (1%)
Total
n=185
46 (51%)
45 (50%)
31 (12-73)
72 (39%)
113 (61%)
95 (51%)
Dark Iris Color: n (%)
49 (52%)
45 (48%)
90 (49%)
Light Iris Color: n (%)
Note: 14 pediatric subjects 12-17years old were enrolled in the trial; Race is more than 100% given subjects could check more than one category.
144 (78%)
33 (18%)
9 (5%)
3 (2%)
Ocuphire
PHARMAView entire presentation