Bluejay Investor Presentation Deck
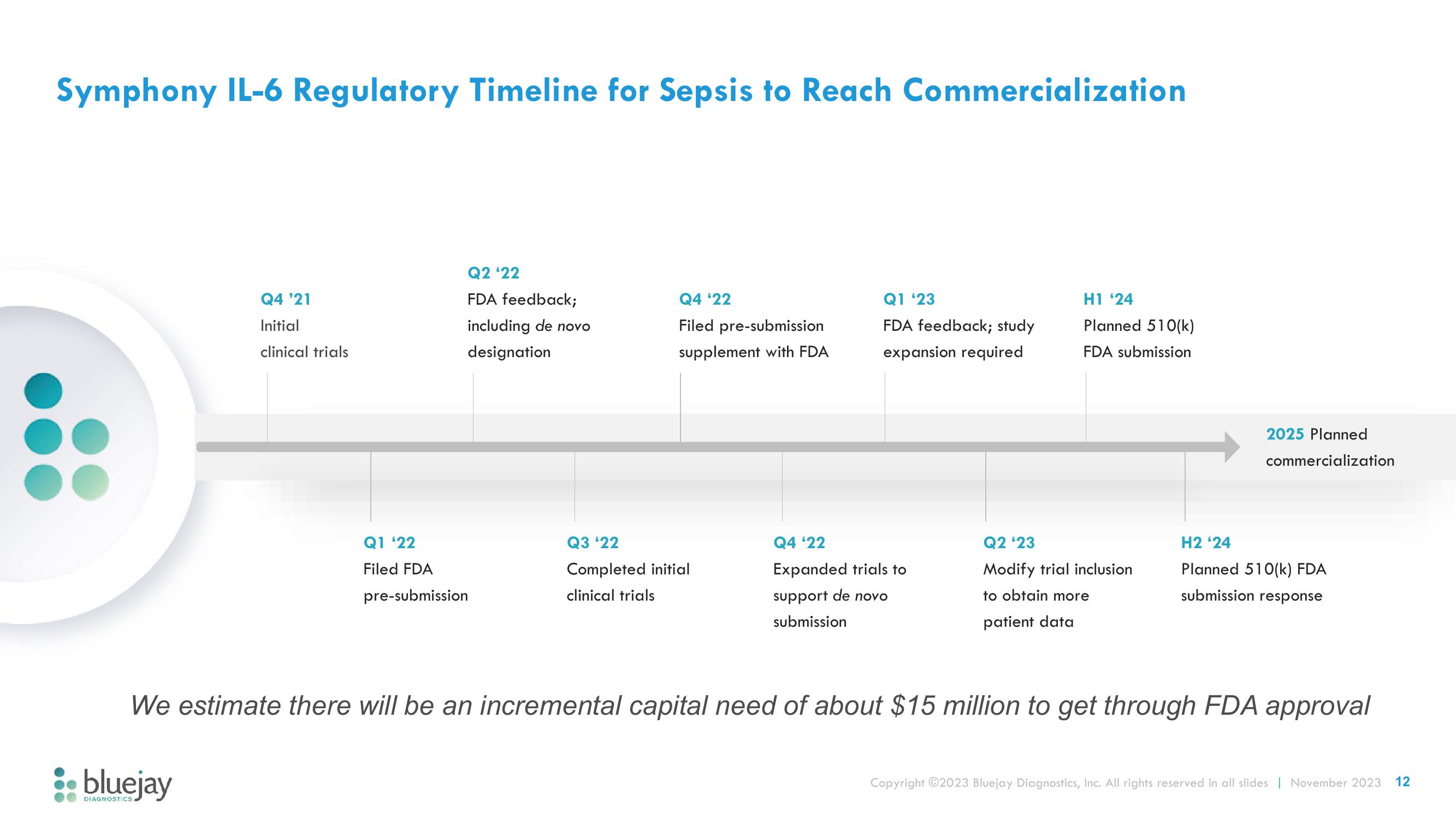
Symphony IL-6 Regulatory Timeline for Sepsis to Reach Commercialization
●●●
●●
Q4 '21
Initial
clinical trials
bluejay
DIAGNOSTICS
Q2 '22
FDA feedback;
including de novo
designation
Q1 '22
Filed FDA
pre-submission
Q4 '22
Filed pre-submission
supplement with FDA
Q3 '22
Completed initial
clinical trials
Q1 '23
FDA feedback; study
expansion required
Q4 '22
Expanded trials to
support de novo
submission
H1 24
Planned 510(k)
FDA submission
Q2 '23
Modify trial inclusion
to obtain more
patient data
2025 Planned
commercialization
H2 '24
Planned 510(k) FDA
submission response
We estimate there will be an incremental capital need of about $15 million to get through FDA approval
Copyright ©2023 Bluejay Diagnostics, Inc. All rights reserved in all slides | November 2023
12View entire presentation