Immix Biopharma Investor Presentation Deck
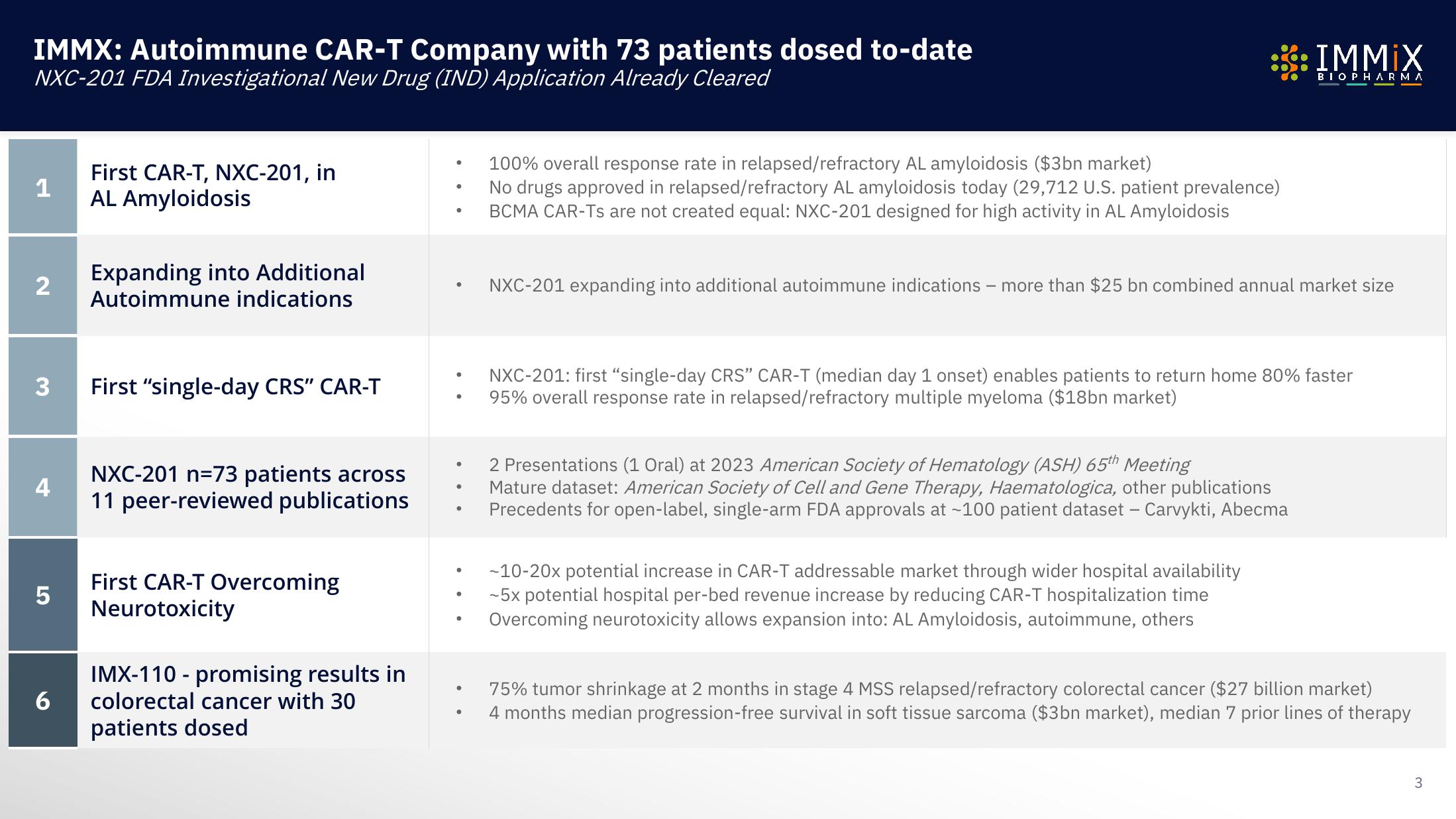
IMMX: Autoimmune CAR-T Company with 73 patients dosed to-date
NXC-201 FDA Investigational New Drug (IND) Application Already Cleared
1
2
3
4
5
6
First CAR-T, NXC-201, in
AL Amyloidosis
Expanding into Additional
Autoimmune indications
First "single-day CRS" CAR-T
NXC-201 n=73 patients across
11 peer-reviewed publications
First CAR-T Overcoming
Neurotoxicity
IMX-110 - promising results in
colorectal cancer with 30
patients dosed
●
●
●
.
●●●
IMMIX
S BIOPHARMA
100% overall response rate in relapsed/refractory AL amyloidosis ($3bn market)
No drugs approved in relapsed/refractory AL amyloidosis today (29,712 U.S. patient prevalence)
BCMA CAR-Ts are not created equal: NXC-201 designed for high activity in AL Amyloidosis
NXC-201 expanding into additional autoimmune indications - more than $25 bn combined annual market size
NXC-201: first "single-day CRS" CAR-T (median day 1 onset) enables patients to return home 80% faster
95% overall response rate in relapsed/refractory multiple myeloma ($18bn market)
2 Presentations (1 Oral) at 2023 American Society of Hematology (ASH) 65th Meeting
Mature dataset: American Society of Cell and Gene Therapy, Haematologica, other publications
Precedents for open-label, single-arm FDA approvals at ~100 patient dataset - Carvykti, Abecma
-10-20x potential increase in CAR-T addressable market through wider hospital availability
-5x potential hospital per-bed revenue increase by reducing CAR-T hospitalization time
Overcoming neurotoxicity allows expansion into: AL Amyloidosis, autoimmune, others
75% tumor shrinkage at 2 months in stage 4 MSS relapsed/refractory colorectal cancer ($27 billion market)
4 months median progression-free survival in soft tissue sarcoma ($3bn market), median 7 prior lines of therapy
3View entire presentation