BioNTech Investor Day Presentation Deck
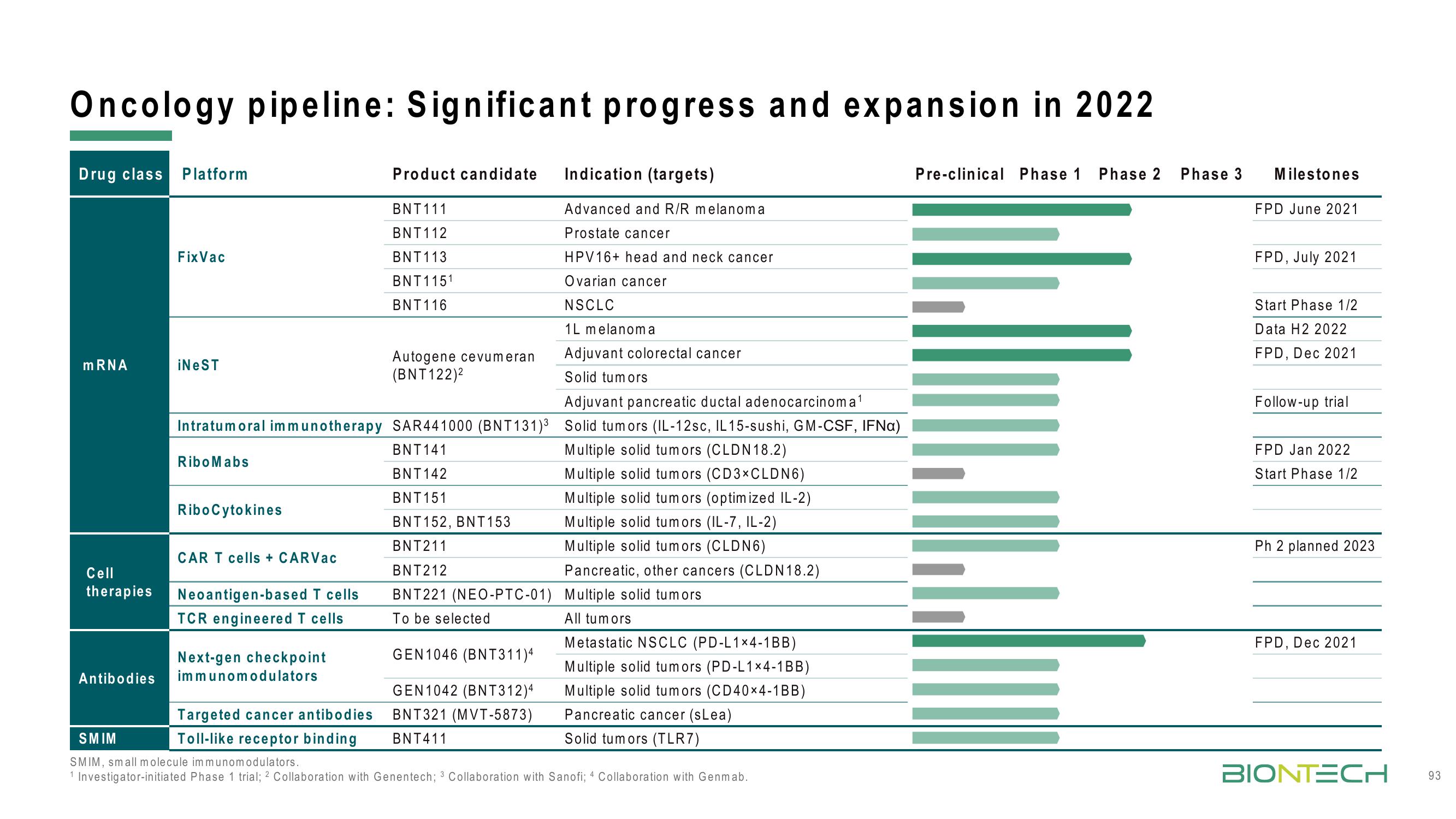
Oncology pipeline: Significant progress and expansion in 2022
Drug class Platform
mRNA
Cell
therapies
Antibodies
Fix Vac
iNeST
RiboMabs
RiboCytokines
CAR T cells + CARVac
Neoantigen-based T cells
TCR engineered T cells
Next-gen checkpoint
immunomodulators
Product candidate Indication (targets)
Adjuvant pancreatic ductal adenocarcinoma ¹
Intratum oral immunotherapy SAR441000 (BNT131)³ Solid tumors (IL-12sc, IL 15-sushi, GM-CSF, IFNa)
BNT141
Multiple solid tumors (CLDN 18.2)
BNT142
BNT 151
BNT152, BNT153
BNT211
BNT212
BNT221 (NEO-PTC-01)
To be selected
Multiple solid tumors (CD3×CLDN6)
Multiple solid tumors (optimized IL-2)
Multiple solid tumors (IL-7, IL-2)
Multiple solid tumors (CLDN6)
Pancreatic, other cancers (CLDN18.2)
Multiple solid tumors
GEN1046 (BNT311)4
GEN1042 (BNT312)4
BNT321 (MVT-5873)
BNT411
Targeted cancer antibodies
Toll-like receptor binding
BNT111
BNT112
BNT113
BNT 1151
BNT 116
Autogene cevum eran
(BNT122)²
Advanced and R/R melanoma
Prostate cancer
HPV16+ head and neck cancer
Ovarian cancer
NSCLC
1L melanoma
Adjuvant colorectal cancer
Solid tumors
All tumors
Metastatic NSCLC (PD-L1x4-1BB)
Multiple solid tumors (PD-L1×4-1BB)
Multiple solid tumors (CD40×4-1BB)
Pancreatic cancer (sLea)
Solid tumors (TLR7)
SMIM
SMIM, small molecule immunomodulators.
1 Investigator-initiated Phase 1 trial; 2 Collaboration with Genentech; 3 Collaboration with Sanofi; 4 Collaboration with Genmab.
Pre-clinical Phase 1 Phase 2 Phase 3
IMAJU
Milestones
FPD June 2021
FPD, July 2021
Start Phase 1/2
Data H2 2022
FPD, Dec 2021
Follow-up trial
FPD Jan 2022
Start Phase 1/2
Ph 2 planned 2023
FPD, Dec 2021
BIONTECH
93View entire presentation