Ocuphire Pharma Investor Updates
P
9
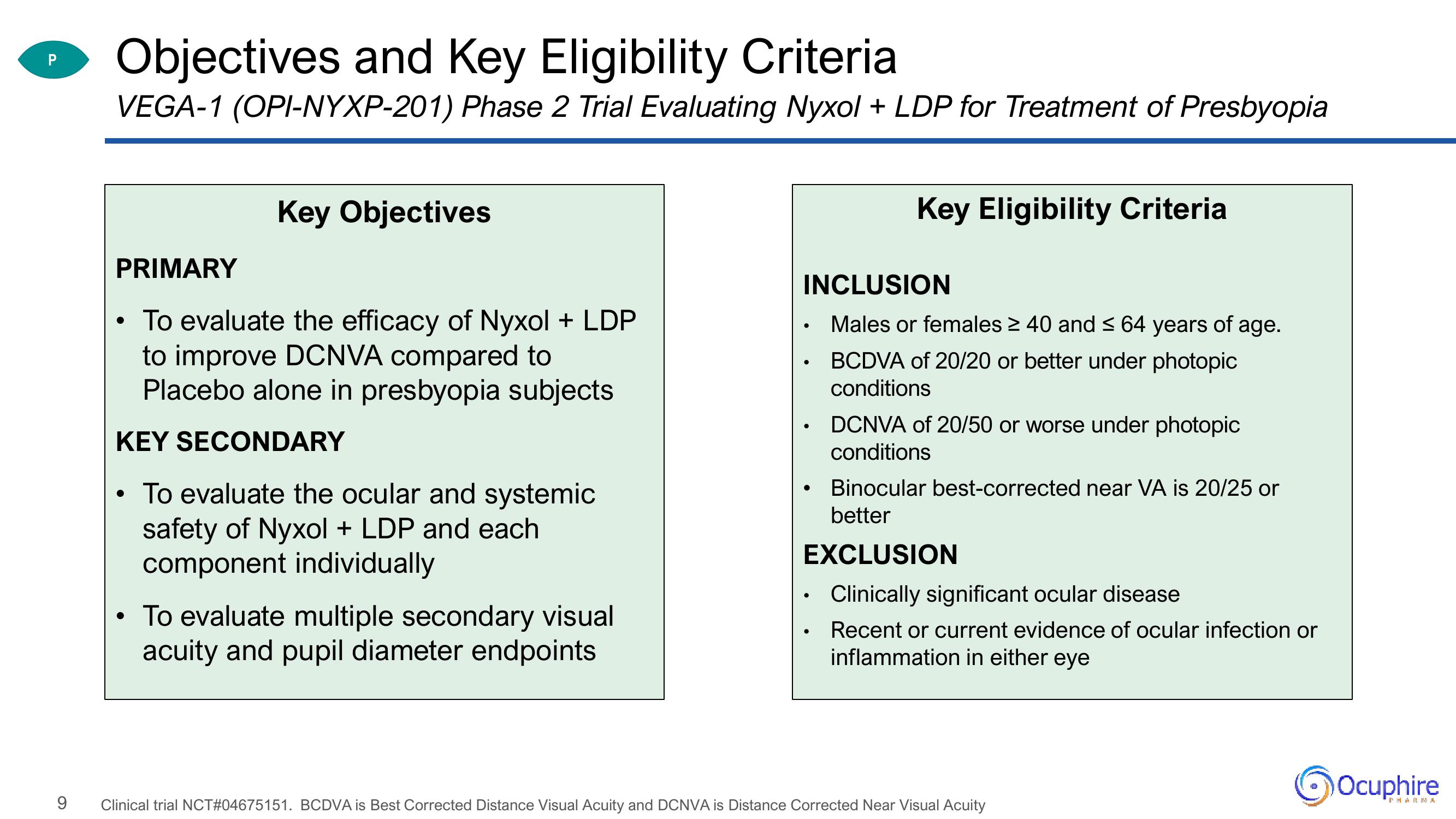
Objectives and Key Eligibility Criteria
VEGA-1 (OPI-NYXP-201) Phase 2 Trial Evaluating Nyxol + LDP for Treatment of Presbyopia
Key Objectives
PRIMARY
To evaluate the efficacy of Nyxol + LDP
to improve DCNVA compared to
Placebo alone in presbyopia subjects
●
KEY SECONDARY
• To evaluate the ocular and systemic
safety of Nyxol + LDP and each
component individually
• To evaluate multiple secondary visual
acuity and pupil diameter endpoints
Key Eligibility Criteria
INCLUSION
Males or females ≥ 40 and ≤ 64 years of age.
BCDVA of 20/20 or better under photopic
conditions
• DCNVA of 20/50 or worse under photopic
conditions
Binocular best-corrected near VA is 20/25 or
better
EXCLUSION
Clinically significant ocular disease
Recent or current evidence of ocular infection or
inflammation in either eye
Clinical trial NCT#04675151. BCDVA is Best Corrected Distance Visual Acuity and DCNVA is Distance Corrected Near Visual Acuity
Ocuphire
PHARMAView entire presentation