Eleusis SPAC
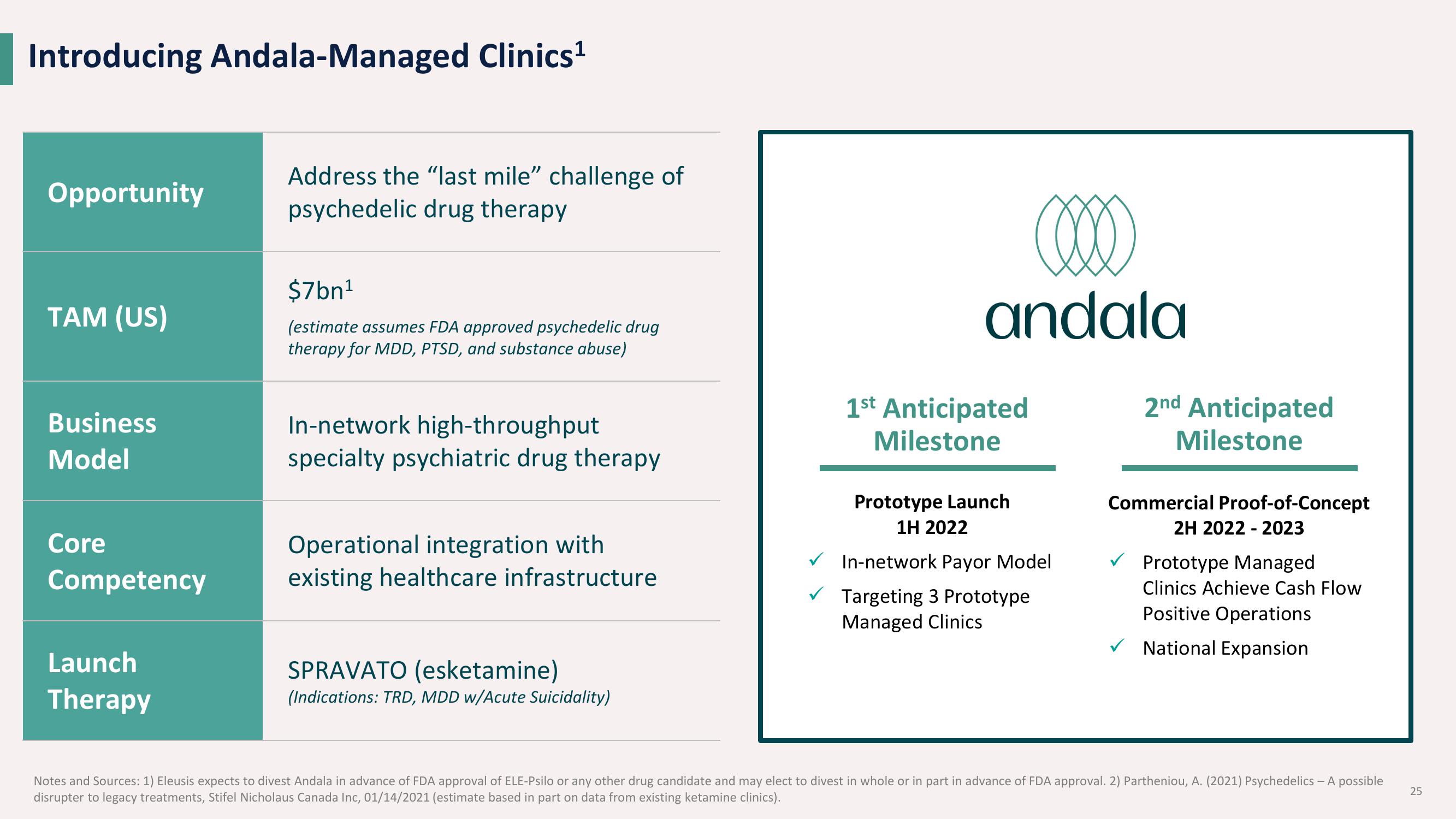
Introducing Andala-Managed Clinics¹
Opportunity
TAM (US)
Business
Model
Core
Competency
Launch
Therapy
Address the "last mile" challenge of
psychedelic drug therapy
$7bn¹
(estimate assumes FDA approved psychedelic drug
therapy for MDD, PTSD, and substance abuse)
In-network high-throughput
specialty psychiatric drug therapy
Operational integration with
existing healthcare infrastructure
SPRAVATO (esketamine)
(Indications: TRD, MDD w/Acute Suicidality)
(♡♡♡♡
andala
1st Anticipated
Milestone
Prototype Launch
1H 2022
✓ In-network Payor Model
✓ Targeting 3 Prototype
Managed Clinics
2nd Anticipated
Milestone
Commercial Proof-of-Concept
2H 2022 2023
✓Prototype Managed
Clinics Achieve Cash Flow
Positive Operations
✓ National Expansion
Notes and Sources: 1) Eleusis expects to divest Andala in advance of FDA approval of ELE-Psilo or any other drug candidate and may elect to divest in whole or in part in advance of FDA approval. 2) Partheniou, A. (2021) Psychedelics - A possible
disrupter to legacy treatments, Stifel Nicholaus Canada Inc, 01/14/2021 (estimate based in part on data from existing ketamine clinics).
25View entire presentation