Immix Biopharma Investor Presentation Deck
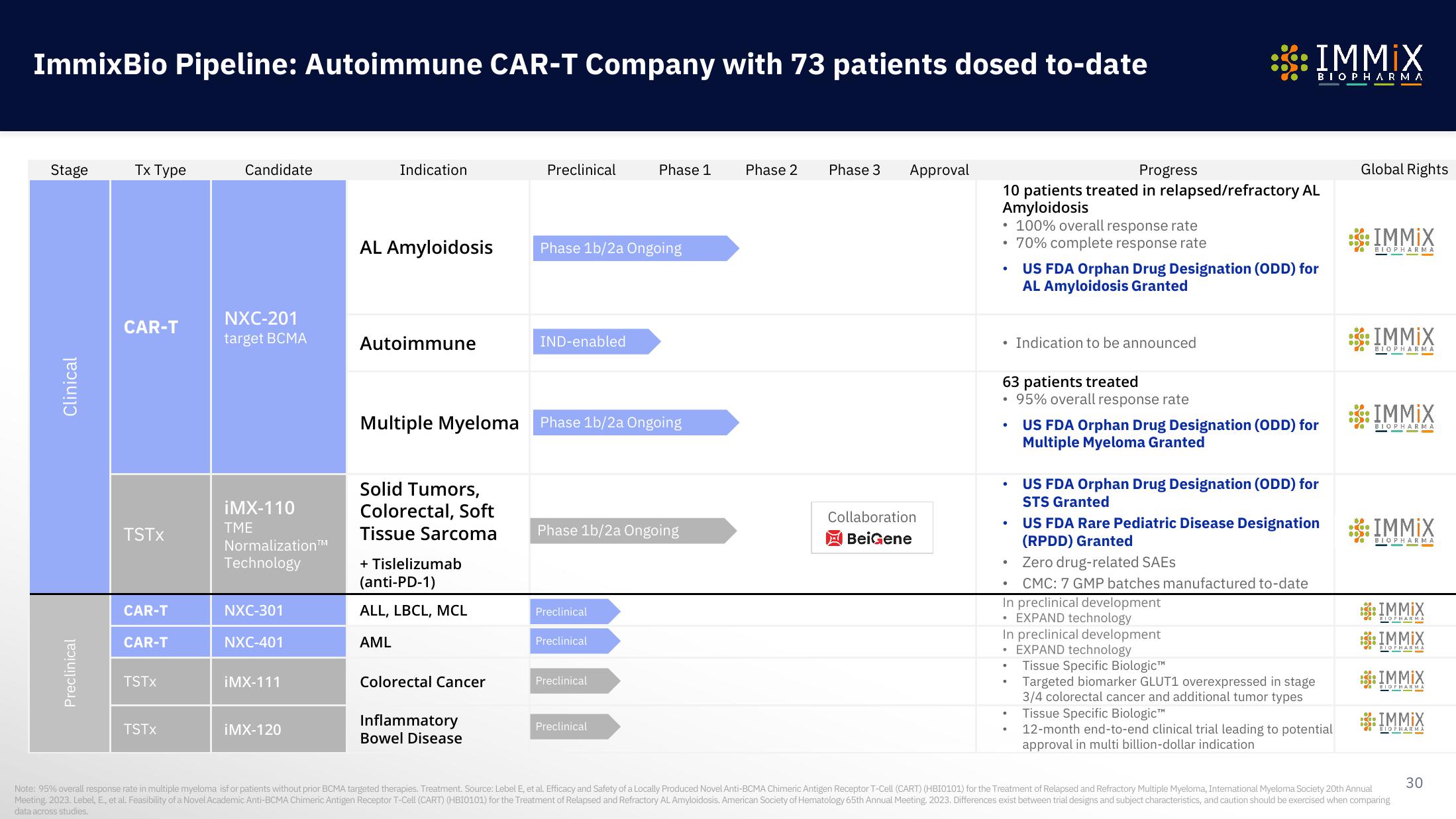
ImmixBio Pipeline: Autoimmune CAR-T Company with 73 patients dosed to-date
Stage
Clinical
Preclinical
Tx Type
CAR-T
TSTX
CAR-T
CAR-T
TSTX
TSTX
Candidate
NXC-201
target BCMA
iMX-110
TME
Normalization™
Technology
NXC-301
NXC-401
iMX-111
iMX-120
Indication
AL Amyloidosis
Autoimmune
Multiple Myeloma
Solid Tumors,
Colorectal, Soft
Tissue Sarcoma
+ Tislelizumab
(anti-PD-1)
ALL, LBCL, MCL
AML
Colorectal Cancer
Inflammatory
Bowel Disease
Preclinical
Phase 1b/2a Ongoing
IND-enabled
Phase 1b/2a Ongoing
Phase 1b/2a Ongoing
Preclinical
Phase 1
Preclinical
Preclinical
Preclinical
Phase 2 Phase 3
Approval
Collaboration
BeiGene
Progress
10 patients treated in relapsed/refractory AL
Amyloidosis
• 100% overall response rate
• 70% complete response rate
• Indication to be announced
63 patients treated
●
95% overall response rate
●
●
●
US FDA Orphan Drug Designation (ODD) for
AL Amyloidosis Granted
●
●●●
IMMİX
S BIOPHARMA
US FDA Orphan Drug Designation (ODD) for
Multiple Myeloma Granted
US FDA Orphan Drug Designation (ODD) for
STS Granted
US FDA Rare Pediatric Disease Designation
(RPDD) Granted
Zero drug-related SAES
CMC: 7 GMP batches manufactured to-date
In preclinical development
EXPAND technology
In preclinical development
• EXPAND technology
Tissue Specific Biologic™
Targeted biomarker GLUT1 overexpressed in stage
3/4 colorectal cancer and additional tumor types
Tissue Specific Biologic™
12-month end-to-end clinical trial leading to potential
approval in multi billion-dollar indication
Global Rights
•
600
*
IMMIX
fo BIOPHARMA
00
•
**
***
do
IMMIX
BIOPHARMA
**
...
+4
do
IMMIX
BIOPHARMA
IMMIX
BIOPHARMA
IMMIX
BIOPHARMA
IMMIX
BIOPHARMA
IMMIX
BIOPHARMA
BIOPHARMA
Note: 95% overall response rate in multiple myeloma isf or patients without prior BCMA targeted therapies. Treatment. Source: Lebel E, et al. Efficacy and Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HB10101) for the Treatment of Relapsed and Refractory Multiple Myeloma, International Myeloma Society 20th Annual
Meeting. 2023. Lebel, E., et al. Feasibility of a Novel Academic Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory AL Amyloidosis. American Society of Hematology 65th Annual Meeting. 2023. Differences exist between trial designs and subject characteristics, and caution should be exercised when comparing
data across studies.
30View entire presentation