Ocuphire Pharma Results
Panorama Study Further Emphasizes Need for Proactive Treatment of NPDR
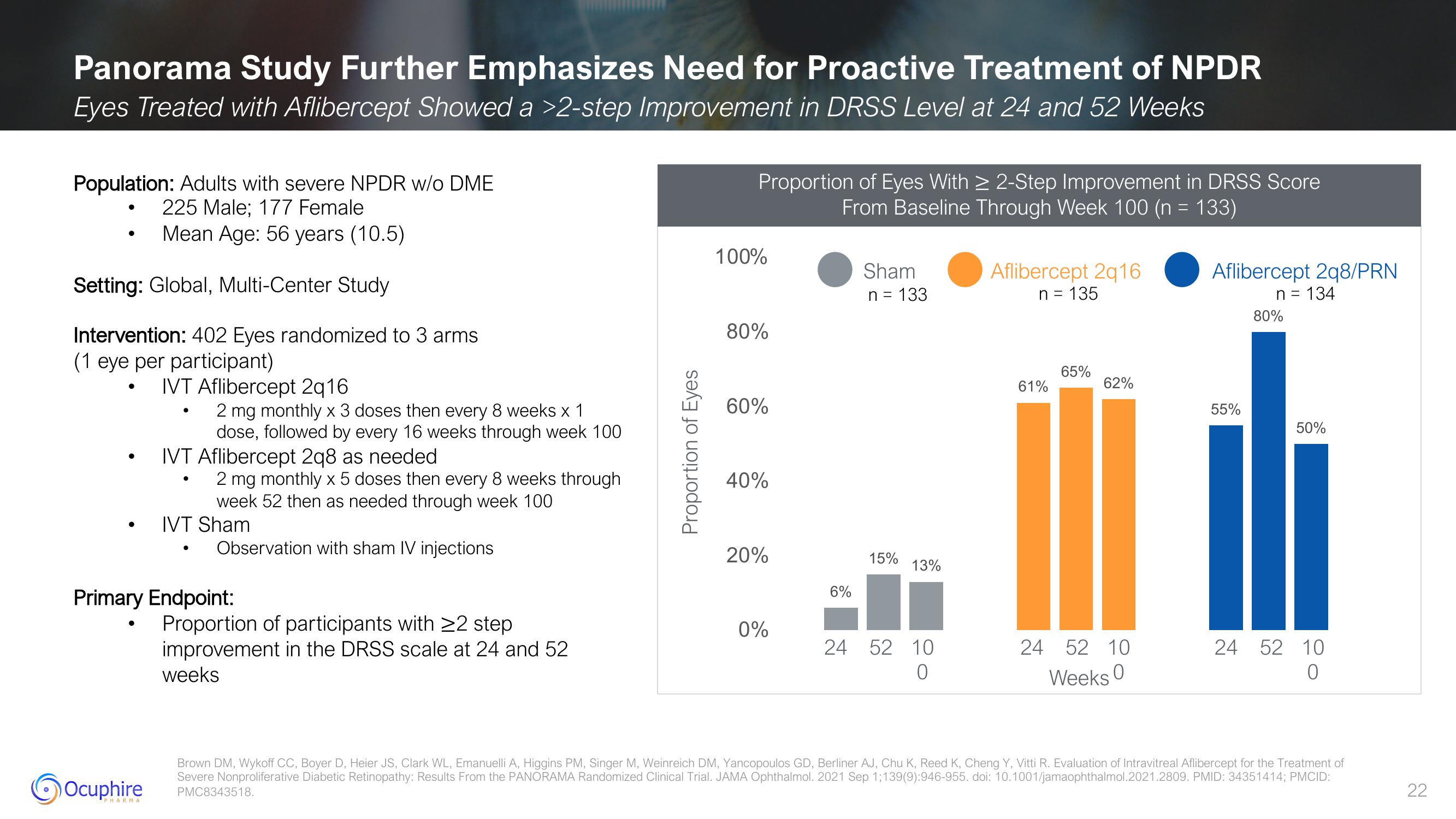
Eyes Treated with Aflibercept Showed a >2-step Improvement in DRSS Level at 24 and 52 Weeks
Population: Adults with severe NPDR w/o DME
225 Male; 177 Female
Mean Age: 56 years (10.5)
Setting: Global, Multi-Center Study
Intervention: 402 Eyes randomized to 3 arms
(1 eye per participant)
●
IVT Aflibercept 2q16
Ocuphire
PHARMA
●
2 mg monthly x 3 doses then every 8 weeks x 1
dose, followed by every 16 weeks through week 100
IVT Aflibercept 2q8 as needed
2 mg monthly x 5 doses then every 8 weeks through
week 52 then as needed through week 100
Observation with sham IV injections
Primary Endpoint:
IVT Sham
Proportion of participants with ≥2 step
improvement in the DRSS scale at 24 and 52
weeks
Proportion of Eyes
Proportion of Eyes With ≥ 2-Step Improvement in DRSS Score
From Baseline Through Week 100 (n = 133)
100%
80%
60%
40%
20%
0%
6%
Sham
n = 133
15%
13%
24 52 10
0
Aflibercept 2q16
n = 135
61%
65%
62%
24 52 10
Weeks 0
Aflibercept 2q8/PRN
n = 134
55%
80%
50%
24 52 10
0
Brown DM, Wykoff CC, Boyer D, Heier JS, Clark WL, Emanuelli A, Higgins PM, Singer M, Weinreich DM, Yancopoulos GD, Berliner AJ, Chu K, Reed K, Cheng Y, Vitti R. Evaluation of Intravitreal Aflibercept for the Treatment of
Severe Nonproliferative Diabetic Retinopathy: Results From the PANORAMA Randomized Clinical Trial. JAMA Ophthalmol. 2021 Sep 1;139(9):946-955. doi: 10.1001/jamaophthalmol.2021.2809. PMID: 34351414; PMCID:
PMC8343518.
22View entire presentation