Dare Bioscience Investor Presentation Deck
DARE-VVA1 - Proof of Concept
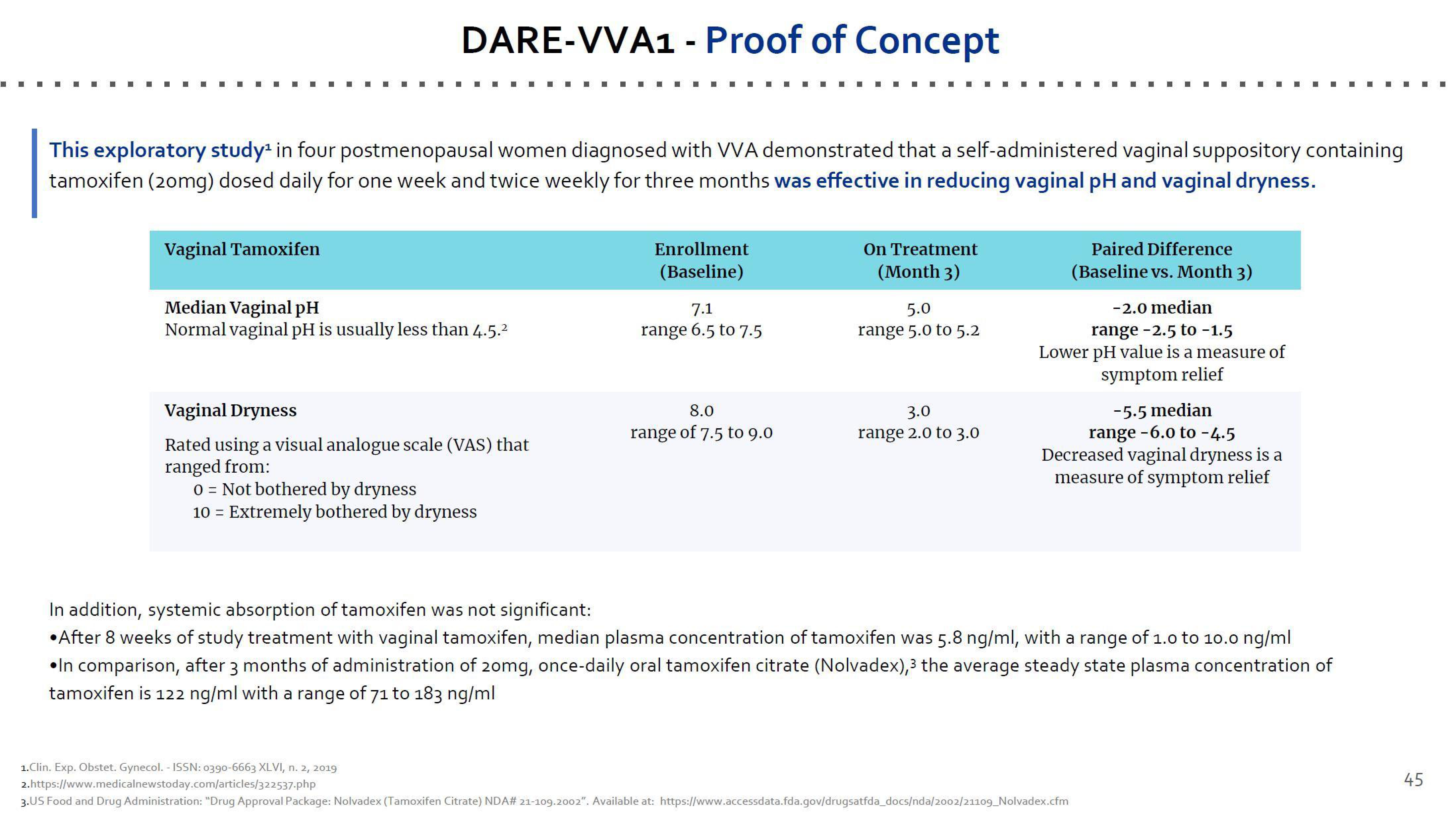
This exploratory study¹ in four postmenopausal women diagnosed with VVA demonstrated that a self-administered vaginal suppository containing
tamoxifen (20mg) dosed daily for one week and twice weekly for three months was effective in reducing vaginal pH and vaginal dryness.
Vaginal Tamoxifen
Median Vaginal pH
Normal vaginal pH is usually less than 4.5.²
Vaginal Dryness
Rated using a visual analogue scale (VAS) that
ranged from:
0 Not bothered by dryness
10 = Extremely bothered by dryness
Enrollment
(Baseline)
7.1
range 6.5 to 7.5
8.0
range of 7.5 to 9.0
On Treatment
(Month 3)
5.0
range 5.0 to 5.2
3.0
range 2.0 to 3.0
Paired Difference
(Baseline vs. Month 3)
-2.0 median
range -2.5 to -1.5
Lower pH value is a measure of
symptom relief
-5.5 median
range -6.0 to -4.5
Decreased vaginal dryness is a
measure of symptom relief
In addition, systemic absorption of tamoxifen was not significant:
•After 8 weeks of study treatment with vaginal tamoxifen, median plasma concentration of tamoxifen was 5.8 ng/ml, with a range of 1.0 to 10.0 ng/ml
In comparison, after 3 months of administration of 20mg, once-daily oral tamoxifen citrate (Nolvadex),3 the average steady state plasma concentration of
tamoxifen is 122 ng/ml with a range of 71 to 183 ng/ml
1.Clin. Exp. Obstet. Gynecol. - ISSN: 0390-6663 XLVI, n. 2, 2019
2.https://www.medicalnewstoday.com/articles/322537.php
3. US Food and Drug Administration: "Drug Approval Package: Nolvadex (Tamoxifen Citrate) NDA# 21-109.2002". Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21109_Nolvadex.cfm
45View entire presentation