LumiraDx Investor Presentation Deck
1
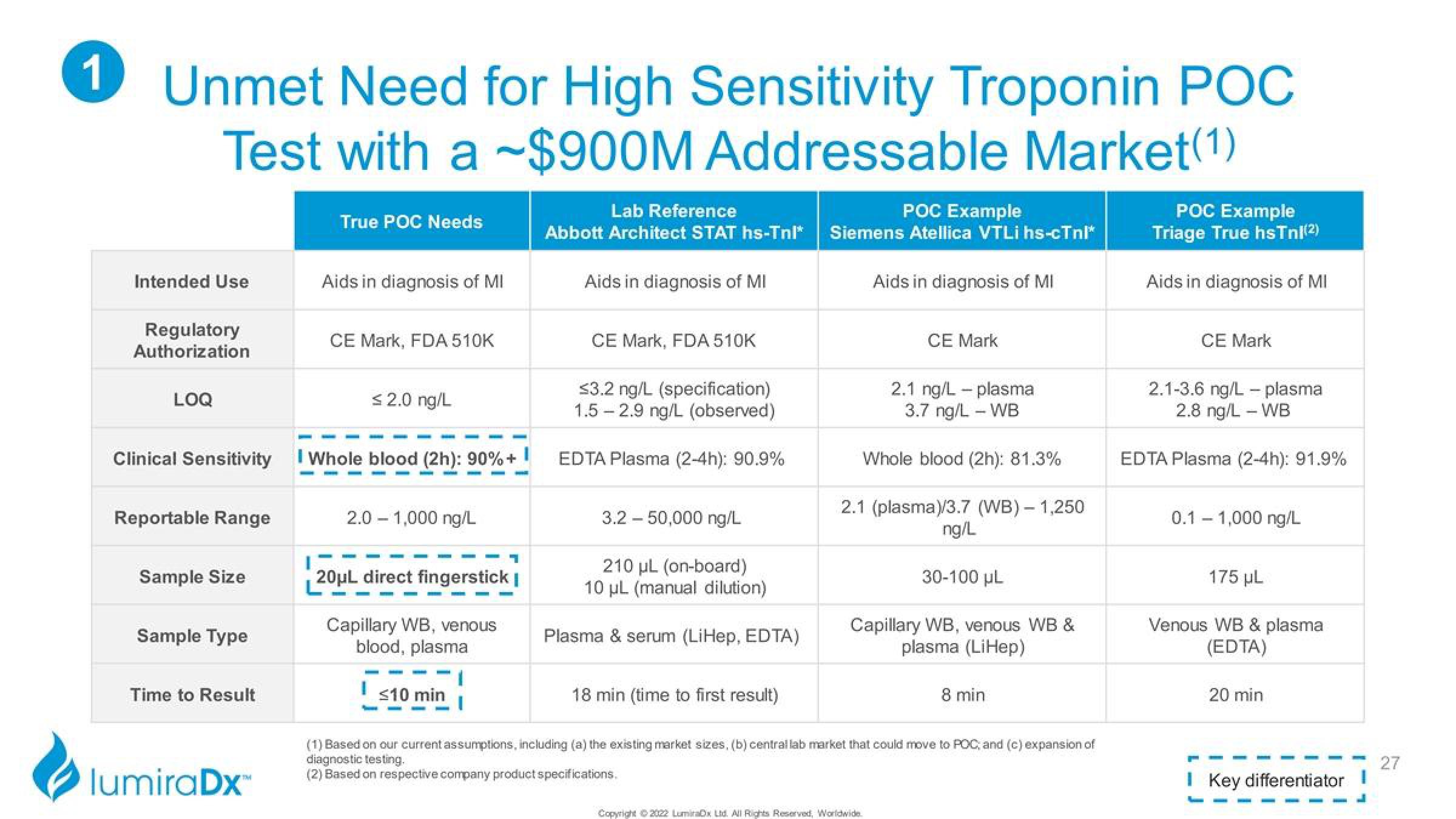
Unmet Need for High Sensitivity Troponin POC
Test with a $900M Addressable Market(1)
Intended Use
Regulatory
Authorization
LOQ
Clinical Sensitivity
Reportable Range
Sample Size
Sample Type
Time to Result
lumiraDx™
True POC Needs
Aids in diagnosis of MI
CE Mark, FDA 510K
s2.0 ng/L
I Whole blood (2h): 90% +
2.0 – 1,000 ng/L
20μL direct fingerstick |
Capillary WB, venous
blood, plasma
≤10 min
Lab Reference
Abbott Architect STAT hs-Tnl*
Aids in diagnosis of MI
CE Mark, FDA 510K
≤3.2 ng/L (specification)
1.5-2.9 ng/L (observed)
EDTA Plasma (2-4h): 90.9%
3.2 – 50,000 ng/L
210 µL (on-board)
10 μL (manual dilution)
Plasma & serum (LiHep, EDTA)
18 min (time to first result)
POC Example
Siemens Atellica VTLi hs-cTnl*
Aids in diagnosis of MI
CE Mark
2.1 ng/L plasma
3.7 ng/L - WB
Whole blood (2h): 81.3%
2.1 (plasma)/3.7 (WB) - 1,250
ng/L
30-100 µL
Capillary WB, venous WB &
plasma (LiHep)
Copyright © 2022 Luminox Ltd. All Rights Reserved, Worldwide
8 min
(1) Based on our current assumptions, including (a) the existing market sizes, (b) central lab market that could move to POC, and (c) expansion of
diagnostic testing.
(2) Based on respective company product specifications.
POC Example
Triage True hsTnl(2)
Aids in diagnosis of MI
CE Mark
2.1-3.6 ng/L - plasma
2.8 ng/L - WB
EDTA Plasma (2-4h): 91.9%
0.1 – 1,000 ng/L
175 µL
Venous WB & plasma
(EDTA)
20 min
Key differentiator
27View entire presentation