Ocuphire Pharma Results
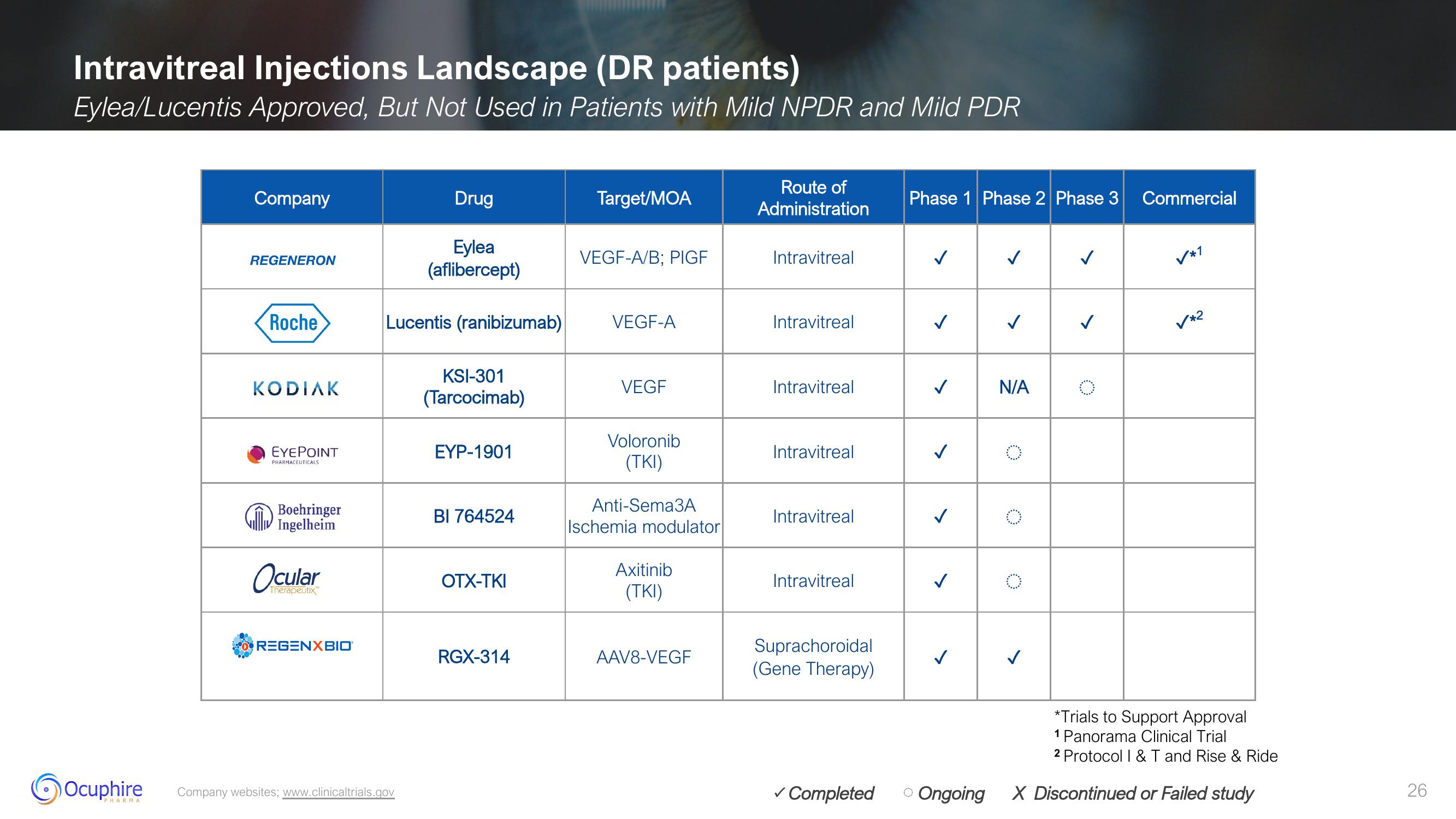
Intravitreal Injections Landscape (DR patients)
Eylea/Lucentis Approved, But Not Used in Patients with Mild NPDR and Mild PDR
Company
REGENERON
Roche
KODIAK
EYEPOINT
PHARMACEUTICALS
Boehringer
Ingelheim
Therapeutix
REGENXBIO
Drug
Eylea
(aflibercept)
Ocuphire Company websites; www.clinicaltrials.gov
PHARMA
Lucentis (ranibizumab) VEGF-A
KSI-301
(Tarcocimab)
EYP-1901
BI 764524
OTX-TKI
Target/MOA
RGX-314
VEGF-A/B; PIGF
VEGF
Voloronib
(TKI)
Anti-Sema3A
Ischemia modulator
Axitinib
(TKI)
AAV8-VEGF
Route of
Administration
Intravitreal
Intravitreal
Intravitreal
Intravitreal
Intravitreal
Intravitreal
Suprachoroidal
(Gene Therapy)
✓ Completed
Phase 1 Phase 2 Phase 3 Commercial
✓
✓
✓
Ongoing
N/A
✓*1
✓*2
*Trials to Support Approval
1 Panorama Clinical Trial
2 Protocol I & T and Rise & Ride
X Discontinued or Failed study
26View entire presentation