Calliditas Therapeutics IPO Presentation Deck
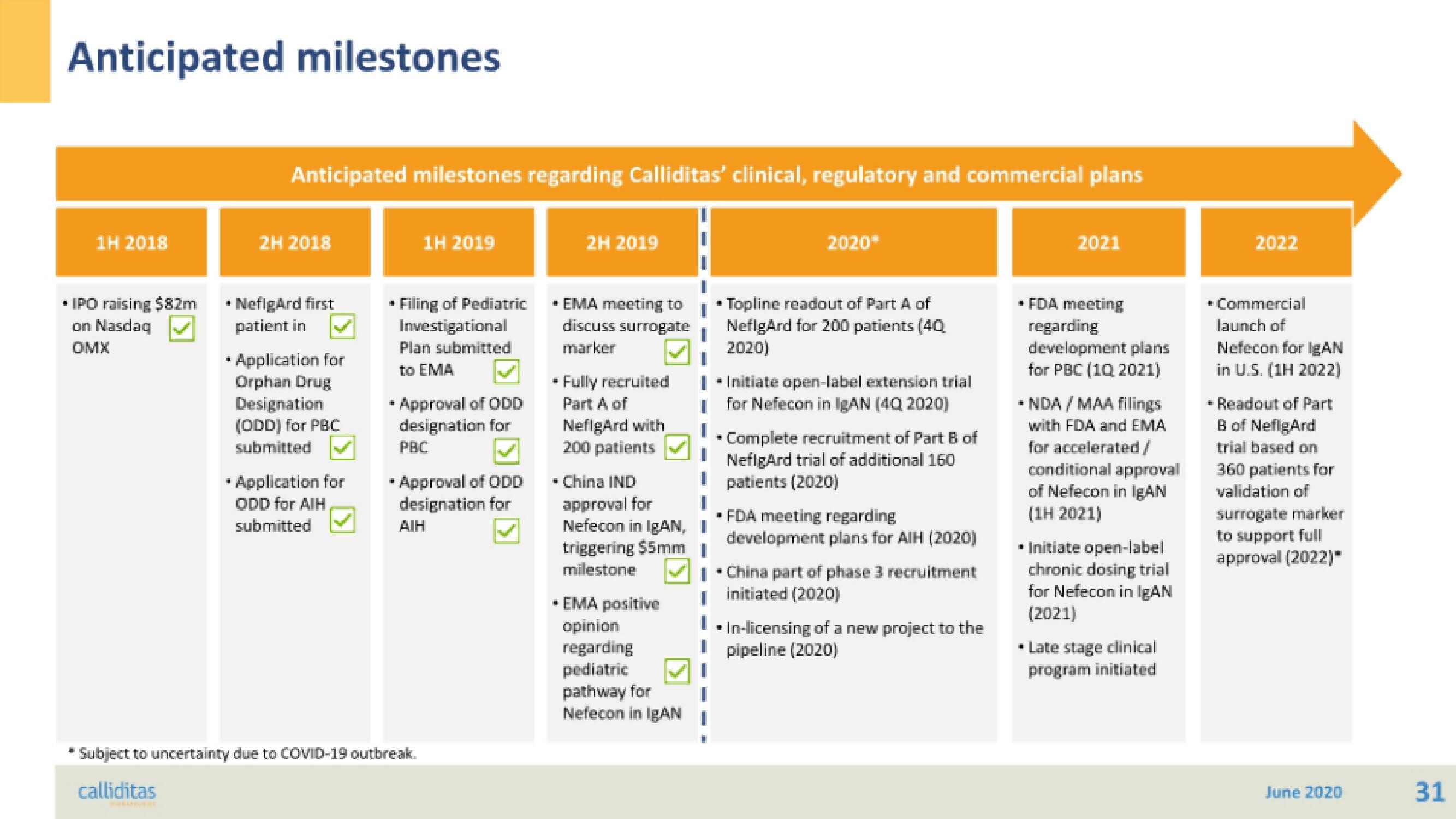
Anticipated milestones
"
1H 2018
IPO raising $82m
on Nasdaq
OMX
Anticipated milestones regarding Calliditas' clinical, regulatory and commercial plans
2H 2018
NefigArd first
patient in
* Application for
Orphan Drug
Designation
(ODD) for PBC
submitted
* Application for
ODD for AIH
submitted
#
H
1H 2019
Filing of Pediatric
Investigational
Plan submitted
to EMA
Approval of ODD
designation for
PBC
• Approval of ODD
designation for
AIH
* Subject to uncertainty due to COVID-19 outbreak.
calliditas
2H 2019
* EMA meeting to
discuss surrogate
marker
=
Fully recruited
Part A of
NefigArd with
200 patients
- China IND
approval for
Nefecon in IgAN, I
triggering $5mm
milestone
* EMA positive
opinion
regarding
pediatric
pathway for
Nefecon in IgAN
* Topline readout of Part A of
NefigArd for 200 patients (40
2020)
I • Initiate open-label extension trial
I
for Nefecon in IgAN (4Q 2020)
■
2020*
■
⠀
Complete recruitment of Part B of
NeflgArd trial of additional 160
patients (2020)
FDA meeting regarding
development plans for AIH (2020)
China part of phase 3 recruitment
initiated (2020)
* In-licensing of a new project to the
pipeline (2020)
2021
• FDA meeting
regarding
development plans
for PBC (1Q 2021)
+NDA / MAA filings
with FDA and EMA
for accelerated/
conditional approval
of Nefecon in IgAN
(1H 2021)
* Initiate open-label
chronic dosing trial
for Nefecon in IgAN
(2021)
* Late stage clinical
program initiated
2022
Commercial
launch of
Nefecon for IgAN
in U.S. (1H 2022)
* Readout of Part
B of NefigArd
trial based on
360 patients for
validation of
surrogate marker
to support full
approval (2022)"
June 2020
31View entire presentation