Immix Biopharma Investor Presentation Deck
Only CAR-T in BCMA-exposed multiple myeloma,
a rapidly growing patient segment (2/2)
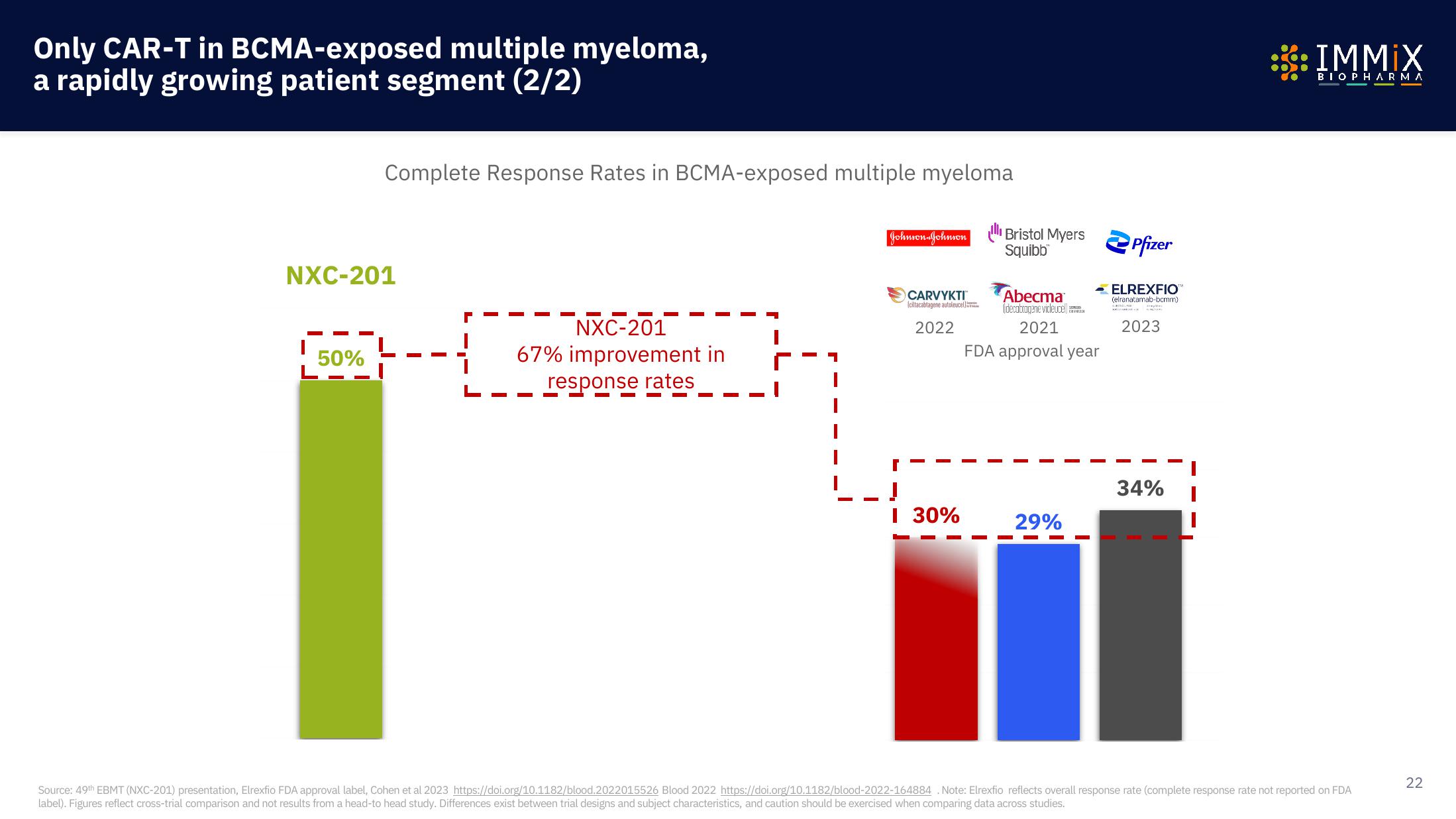
Complete Response Rates in BCMA-exposed multiple myeloma
NXC-201
50%
NXC-201
67% improvement in
response rates
Johnson-Johnson Bristol Myers Pfizer
Squibb
CARVYKTI™
Iciltacablagene autoleuce
2022
I 30%
Abecma
(decabragene vicleuce)
2021
FDA approval year
29%
ELREXFIO
(elranatamab-bcmm)
2023
34%
●●●
IMMIX
S BIOPHARMA
Source: 49th EBMT (NXC-201) presentation, Elrexfio FDA approval label, Cohen et al 2023 https://doi.org/10.1182/blood.2022015526 Blood 2022 https://doi.org/10.1182/blood-2022-164884. Note: Elrexfio reflects overall response rate (complete response rate not reported on FDA
label). Figures reflect cross-trial comparison and not results from a head-to head study. Differences exist between trial designs and subject characteristics, and caution should be exercised when comparing data across studies.
22View entire presentation