Matinas BioPharma Investor Presentation Deck
22
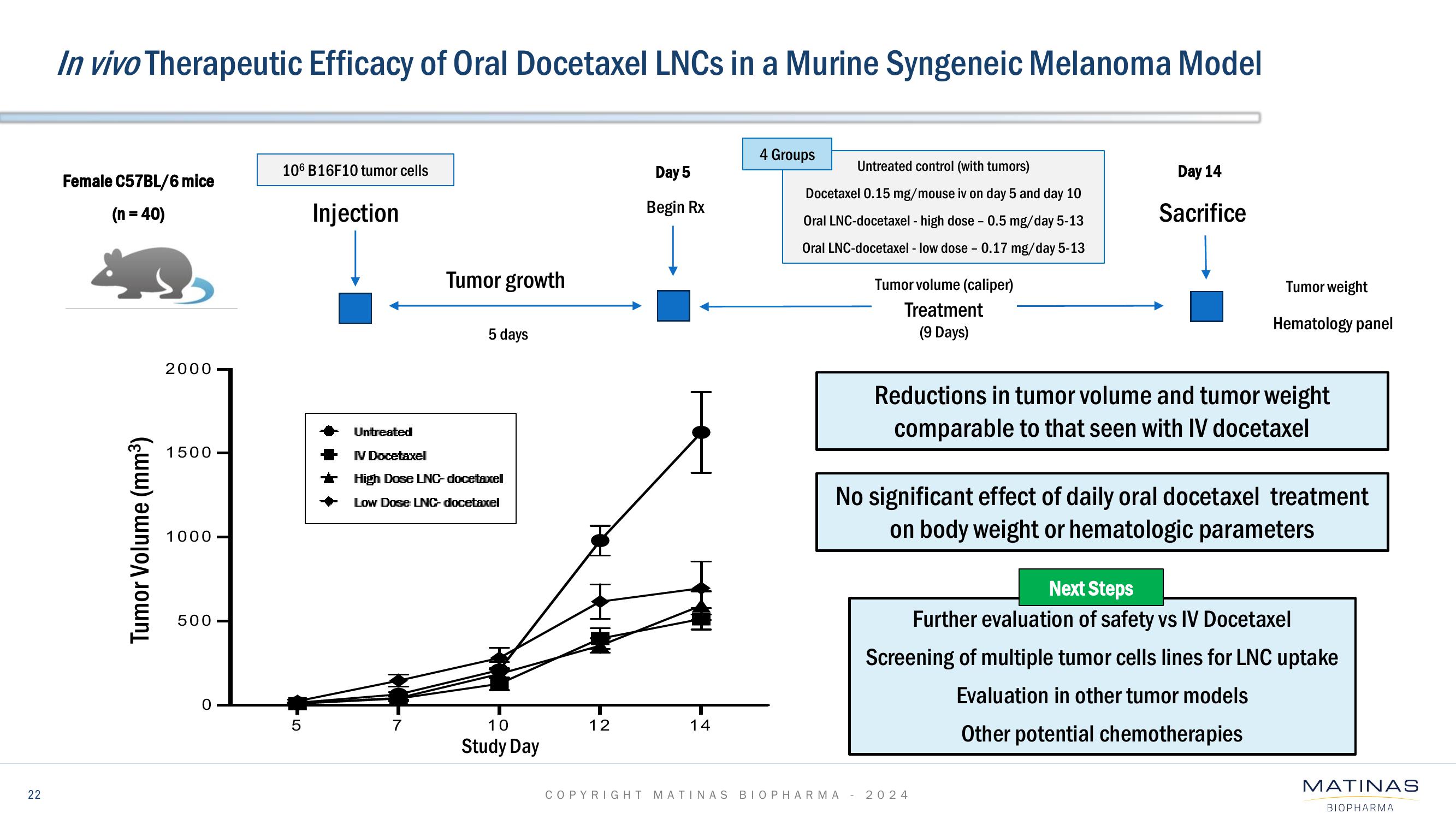
In vivo Therapeutic Efficacy of Oral Docetaxel LNCs in a Murine Syngeneic Melanoma Model
Female C57BL/6 mice
(n = 40)
3
Tumor Volume (mm³)
2000
1500
1000
500
O
106 B16F10 tumor cells
5
Injection
Tumor growth
7
5 days
Untreated
IV Docetaxel
High Dose LNC-docetaxel
Low Dose LNC-docetaxel
10
Study Day
12
Day 5
Begin Rx
+
14
4 Groups
Untreated control (with tumors)
Docetaxel 0.15 mg/mouse iv on day 5 and day 10
Oral LNC-docetaxel - high dose - 0.5 mg/day 5-13
Oral LNC-docetaxel - low dose - 0.17 mg/day 5-13
Tumor volume (caliper)
Treatment
(9 Days)
COPYRIGHT MATINAS BIOPHARMA
Day 14
Sacrifice
Tumor weight
Hematology panel
Reductions in tumor volume and tumor weight
comparable to that seen with IV docetaxel
No significant effect of daily oral docetaxel treatment
on body weight or hematologic parameters
2024
Next Steps
Further evaluation of safety vs IV Docetaxel
Screening of multiple tumor cells lines for LNC uptake
Evaluation in other tumor models
Other potential chemotherapies
MATINAS
BIOPHARMAView entire presentation