Taysha IPO Presentation Deck
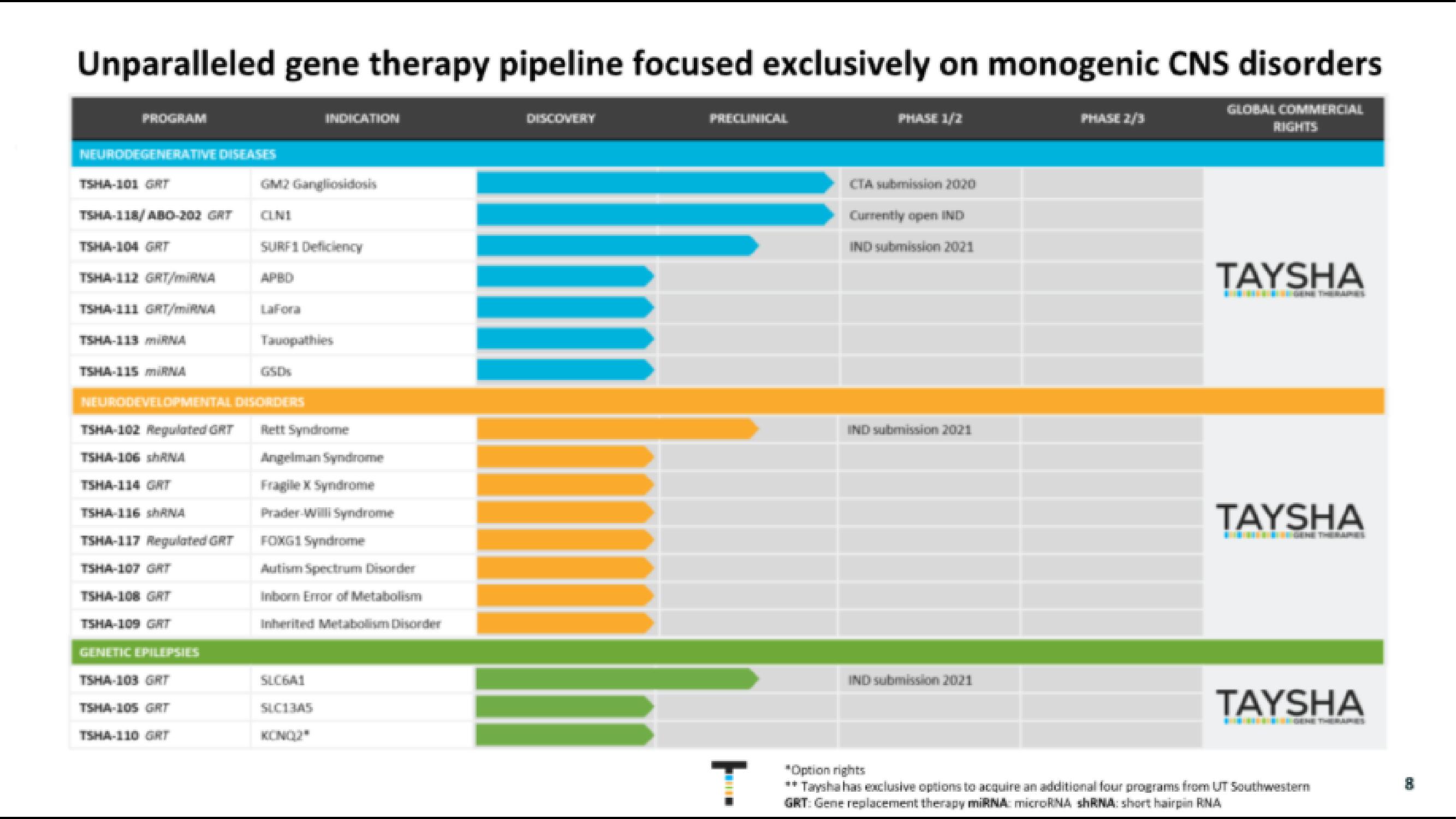
Unparalleled gene therapy pipeline focused exclusively on monogenic CNS disorders
PROGRAM
NEURODEGENERATIVE DISEASES
TSHA-101 GRT
TSHA-118/ ABO-202 GRT
TSHA-104 GRT
TSHA-112 GRT/miRNA
TSHA-111 GRT/MURNA
TSHA-119 MIRNA
TSHA-115 mIRNA
TSHA-102 Regulated GRT
TSHA-106 shANA
TSHA-116 shANA
TSHA-117 Regulated GRT
TSHA-107 GAT
TSHA-108 GRT
TSHA-109 GAT
GENETIC EPILEPSILS
TSHA-103 GRT
TSHA-105 GRT
NEURODEVELOPMENTAL DISORDERS
TSHA-110 GAT
GM2 Gangliosidosis
CLN1
SURF1 Deficiency
Lafora
Tauopathies
GSD
INDICATION
Rett Syndrome
Angelman Syndrome
Fragile X Syndrome
Prader-Willi Syndrome
FOXG1 Syndrome
Autism Spectrum Disorder
Inborn Error of Metabolism
Inherited Metabolism Disorder
SLC6A1
SLC13AS
KONO
DISCOVERY
PRECLINICAL
PHASE 1/2
CTA submission 2020
Currently open IND
IND submission 2021
IND submission 2021
IND submission 2021
PHASE 2/3
GLOBAL COMMERCIAL
RIGHTS
TAYSHA
TAYSHA
TAYSHA
*Option rights
**Taysha has exclusive options to acquire an additional four programs from UT Southwestern
GRT: Gene replacement therapy miRNA: microRNA shRNA: short hairpin RNAView entire presentation