Centessa IPO Presentation Deck
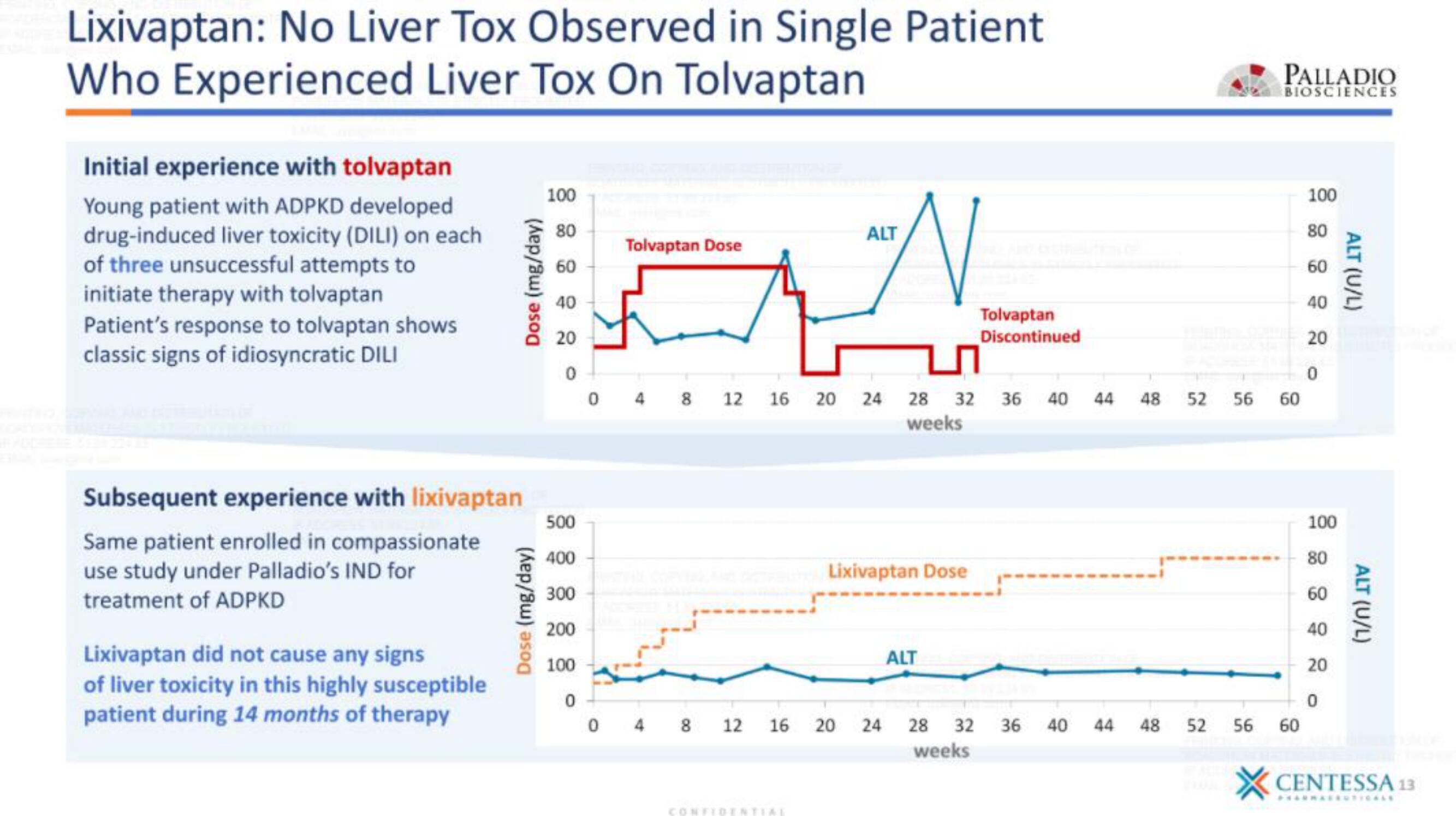
Lixivaptan: No Liver Tox Observed in Single Patient
Who Experienced Liver Tox On Tolvaptan
Initial experience with tolvaptan
Young patient with ADPKD developed
drug-induced liver toxicity (DILI) on each
of three unsuccessful attempts to
initiate therapy with tolvaptan
Patient's response to tolvaptan shows
classic signs of idiosyncratic DILI
Subsequent experience with lixivaptan
Same patient enrolled in compassionate
use study under Palladio's IND for
treatment of ADPKD
Lixivaptan did not cause any signs
of liver toxicity in this highly susceptible
patient during 14 months of therapy
Dose (mg/day)
100
80
60
40
20
0
500
400
300
200
100
0
Tolvaptan Dose
0
4 8 12
048 12 16 20 24 28 32 36 40
weeks
16
Ny
N
20
Lixivaptan Dose
24
Tolvaptan
Discontinued
ALT
28 32 36
weeks
40
44
44
48
48
PALLADIO
BIOSCIENCES
52 56 60
52
56 60
100
80
60
40
20
0
100
80
60
40
20
0
ALT (U/L)
ALT (U/L)
CENTESSA 13View entire presentation