ProSomnus SPAC Presentation Deck
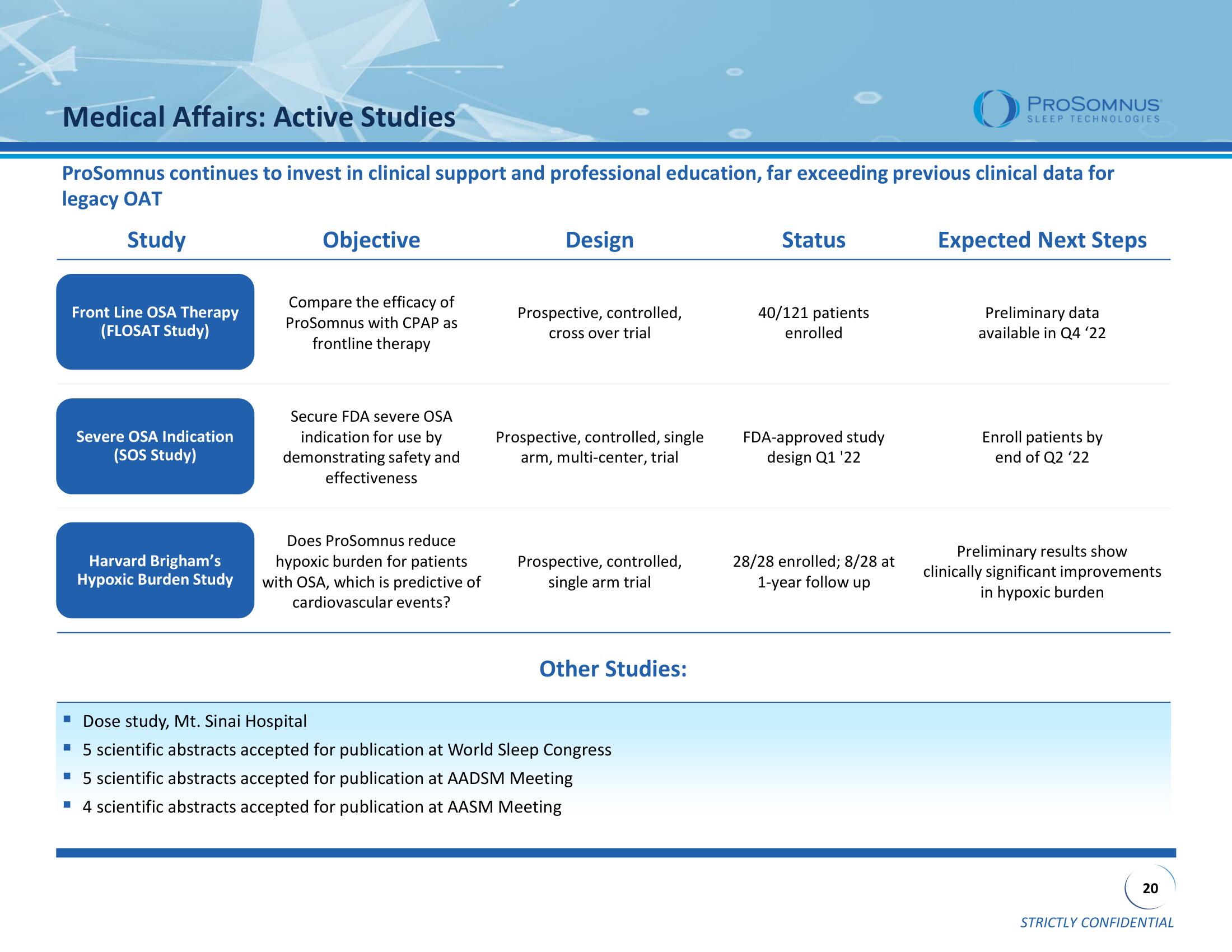
Front Line OSA Therapy
(FLOSAT Study)
Medical Affairs: Active Studies
ProSomnus continues to invest in clinical support and professional education, far exceeding previous clinical data for
legacy OAT
Study
Severe OSA Indication
(SOS Study)
Harvard Brigham's
Hypoxic Burden Study
Objective
Compare the efficacy of
ProSomnus with CPAP as
frontline therapy
Secure FDA severe OSA
indication for use by
demonstrating safety and
effectiveness
Does ProSomnus reduce
hypoxic burden for patients
with OSA, which is predictive of
cardiovascular events?
Design
Prospective, controlled,
cross over trial
Prospective, controlled, single
arm, multi-center, trial
Prospective, controlled,
single arm trial
Other Studies:
Dose study, Mt. Sinai Hospital
▪ 5 scientific abstracts accepted for publication at World Sleep Congress
▪ 5 scientific abstracts accepted for publication at AADSM Meeting
■ 4 scientific abstracts accepted for publication at AASM Meeting
Status
40/121 patients
enrolled
FDA-approved study
design Q1 '22
O
28/28 enrolled; 8/28 at
1-year follow up
PROSOMNUS
SLEEP TECHNOLOGIES
Expected Next Steps
Preliminary data
available in Q4 '22
Enroll patients by
end of Q2 '22
Preliminary results show
clinically significant improvements
in hypoxic burden
20
STRICTLY CONFIDENTIALView entire presentation