MaxCyte Investor Presentation Deck
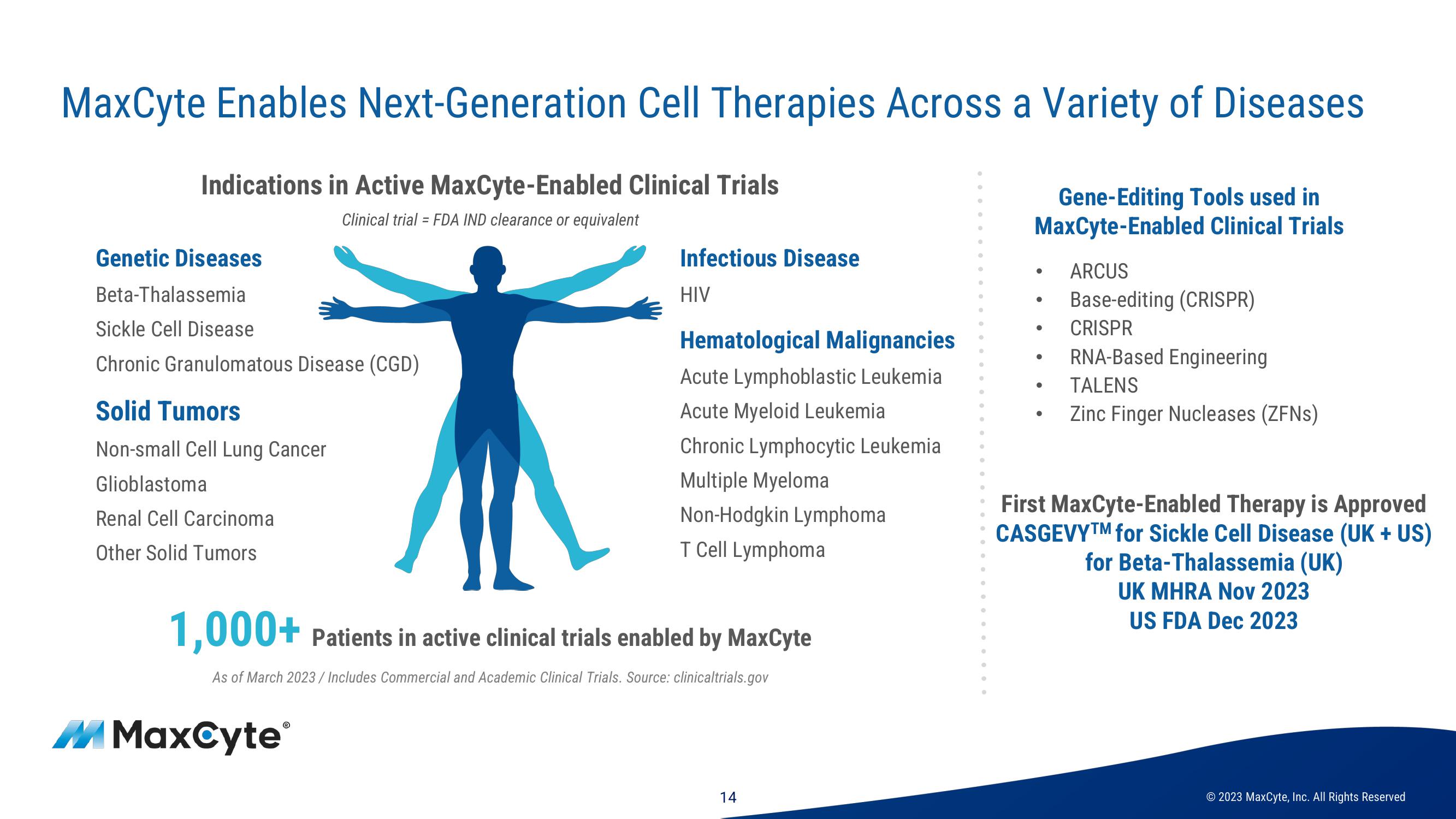
MaxCyte Enables Next-Generation Cell Therapies Across a Variety of Diseases
Indications in Active MaxCyte-Enabled Clinical Trials
Clinical trial = FDA IND clearance or equivalent
Genetic Diseases
Beta-Thalassemia
Sickle Cell Disease
Chronic Granulomatous Disease (CGD)
Solid Tumors
Non-small Cell Lung Cancer
Glioblastoma
Renal Cell Carcinoma
Other Solid Tumors
Infectious Disease
M MaxCyte
HIV
Hematological Malignancies
Acute Lymphoblastic Leukemia
Acute Myeloid Leukemia
Chronic Lymphocytic Leukemia
Multiple Myeloma
Non-Hodgkin Lymphoma
T Cell Lymphoma
1,000+ Patients in active clinical trials enabled by MaxCyte
As of March 2023/ Includes Commercial and Academic Clinical Trials. Source: clinical trials.gov
14
●
●
●
O
●
●
.
Gene-Editing Tools used in
MaxCyte-Enabled Clinical Trials
●
●
●
●
●
ARCUS
Base-editing (CRISPR)
CRISPR
RNA-Based Engineering
TALENS
Zinc Finger Nucleases (ZFNs)
First MaxCyte-Enabled Therapy is Approved
CASGEVYTM for Sickle Cell Disease (UK + US)
for Beta-Thalassemia (UK)
UK MHRA Nov 2023
US FDA Dec 2023
© 2023 MaxCyte, Inc. All Rights ReservedView entire presentation