Immix Biopharma Investor Presentation Deck
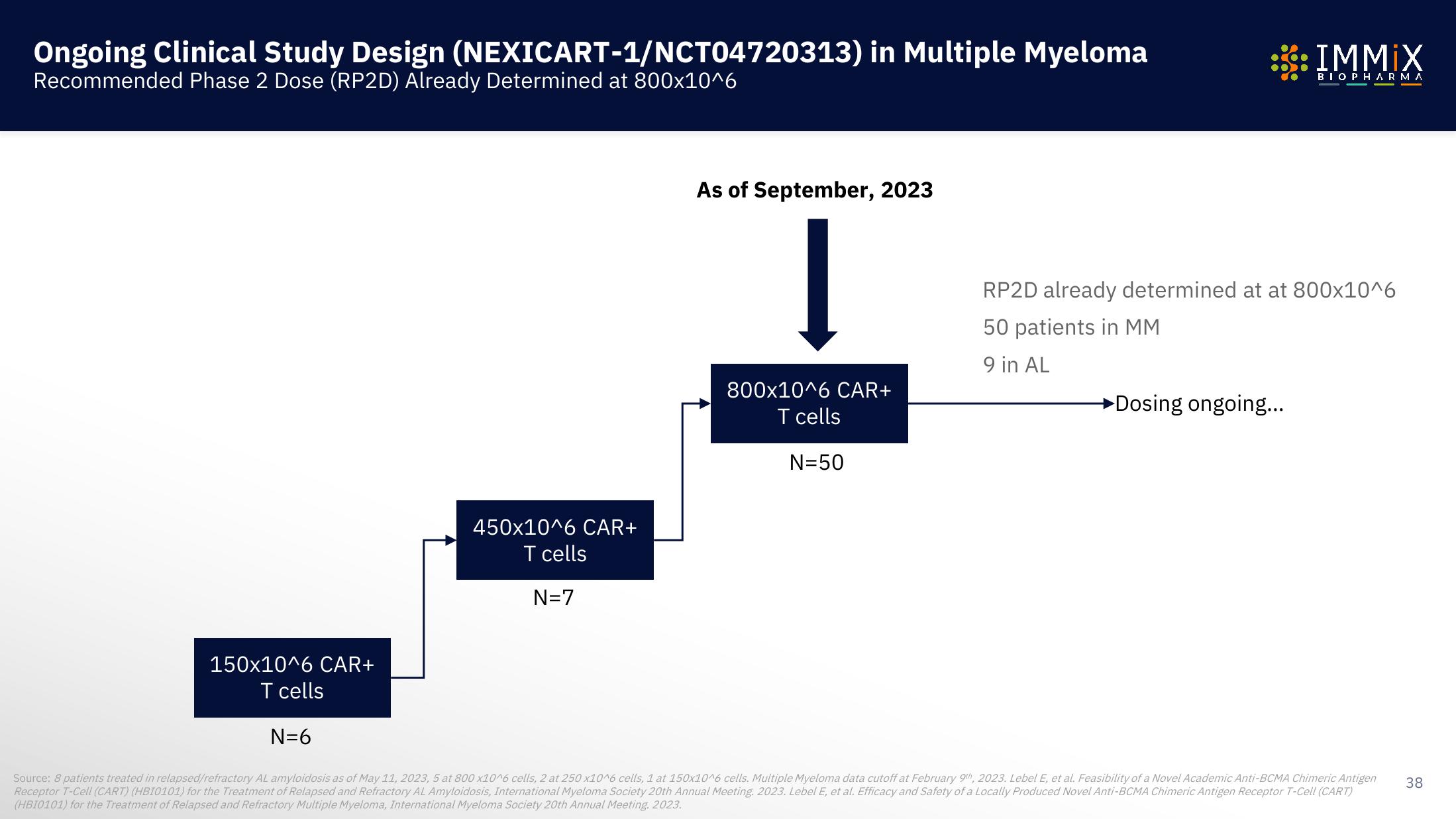
Ongoing Clinical Study Design
Recommended Phase 2 Dose (RP2D) Already Determined at 800x10^6
150x10^6 CAR+
T cells
(NEXICART-1/NCT04720313) in Multiple Myeloma
N=6
450x10^6 CAR+
T cells
N=7
As of September, 2023
800x10^6 CAR+
T cells
N=50
●●●
IMMIX
S BIOPHARMA
RP2D already determined at at 800x10^6
50 patients in MM
9 in AL
►Dosing ongoing...
Source: 8 patients treated in relapsed/refractory AL amyloidosis as of May 11, 2023, 5 at 800 x10^6 cells, 2 at 250 x10^6 cells, 1 at 150x10^6 cells. Multiple Myeloma data cutoff at February 9th, 2023. Lebel E, et al. Feasibility of a Novel Academic Anti-BCMA Chimeric Antigen
Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory AL Amyloidosis, International Myeloma Society 20th Annual Meeting. 2023. Lebel E, et al. Efficacy and Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen Receptor T-Cell (CART)
(HBI0101) for the Treatment of Relapsed and Refractory Multiple Myeloma, International Myeloma Society 20th Annual Meeting. 2023.
38View entire presentation