AstraZeneca Results Presentation Deck
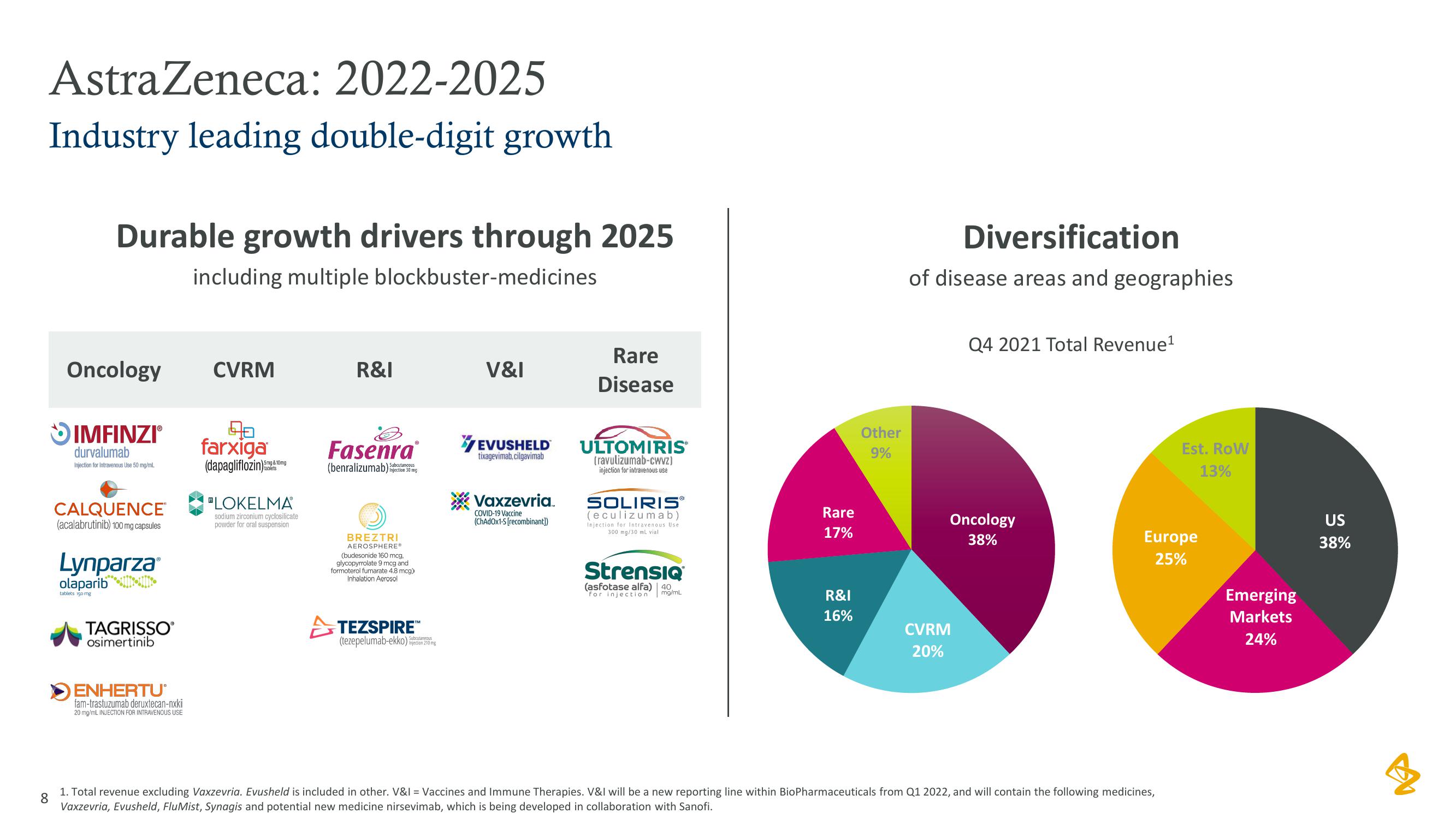
AstraZeneca: 2022-2025
Industry leading double-digit growth
Durable growth drivers through 2025
including multiple blockbuster-medicines
Oncology
SIMFINZIⓇ
durvalumab
Injection for Intravenous Use 50 mg/ml
CALQUENCE®
(acalabrutinib) 100 mg capsules
Lynparza®
olaparib
tablets 150 mg
TAGRISSOⓇ
osimertinib
ENHERTU
fam-trastuzumab deruxtecan-nxki
20 mg/mL INJECTION FOR INTRAVENOUS USE
CVRM
19.0
farxiga
(dapagliflozin)
5mg & 10mg
LOKELMA
sodium zirconium cyclosilicate
powder for oral suspension
R&I
Fasenra
(benralizumab) injection 30 mg
BREZTRI
AEROSPHERE®
(budesonide 160 mcg.
glycopyrrolate 9 mcg and
formoterol fumarate 4.8 mcg)
Inhalation Aerosol
TEZSPIRE™
(tezepelumab-ekko) in 210 mg
V&I
EVUSHELD
tixagevimab, cilgavimab
Vaxzevria
COVID-19 Vaccine
(ChAdOx1-S [recombinant])
Rare
Disease
ULTOMIRIS
[ravulizumab-cwvZ)
injection for intravenous use
SOLIRIS
(eculizumab).
Injection for Intravenous Use
300 mg/30 mL vial
Strensiq
(asfotase alfa) 40,
for injection
mg/mL
Rare
17%
R&I
16%
Other
9%
Diversification
of disease areas and geographies
Q4 2021 Total Revenue¹
Oncology
38%
CVRM
20%
Est. Row
13%
Europe
25%
8
1. Total revenue excluding Vaxzevria. Evusheld is included in other. V&I = Vaccines and Immune Therapies. V&I will be a new reporting line within BioPharmaceuticals from Q1 2022, and will contain the following medicines,
Vaxzevria, Evusheld, FluMist, Synagis and potential new medicine nirsevimab, which is being developed in collaboration with Sanofi.
Emerging
Markets
24%
US
38%
BView entire presentation