Kymera Investor Presentation Deck
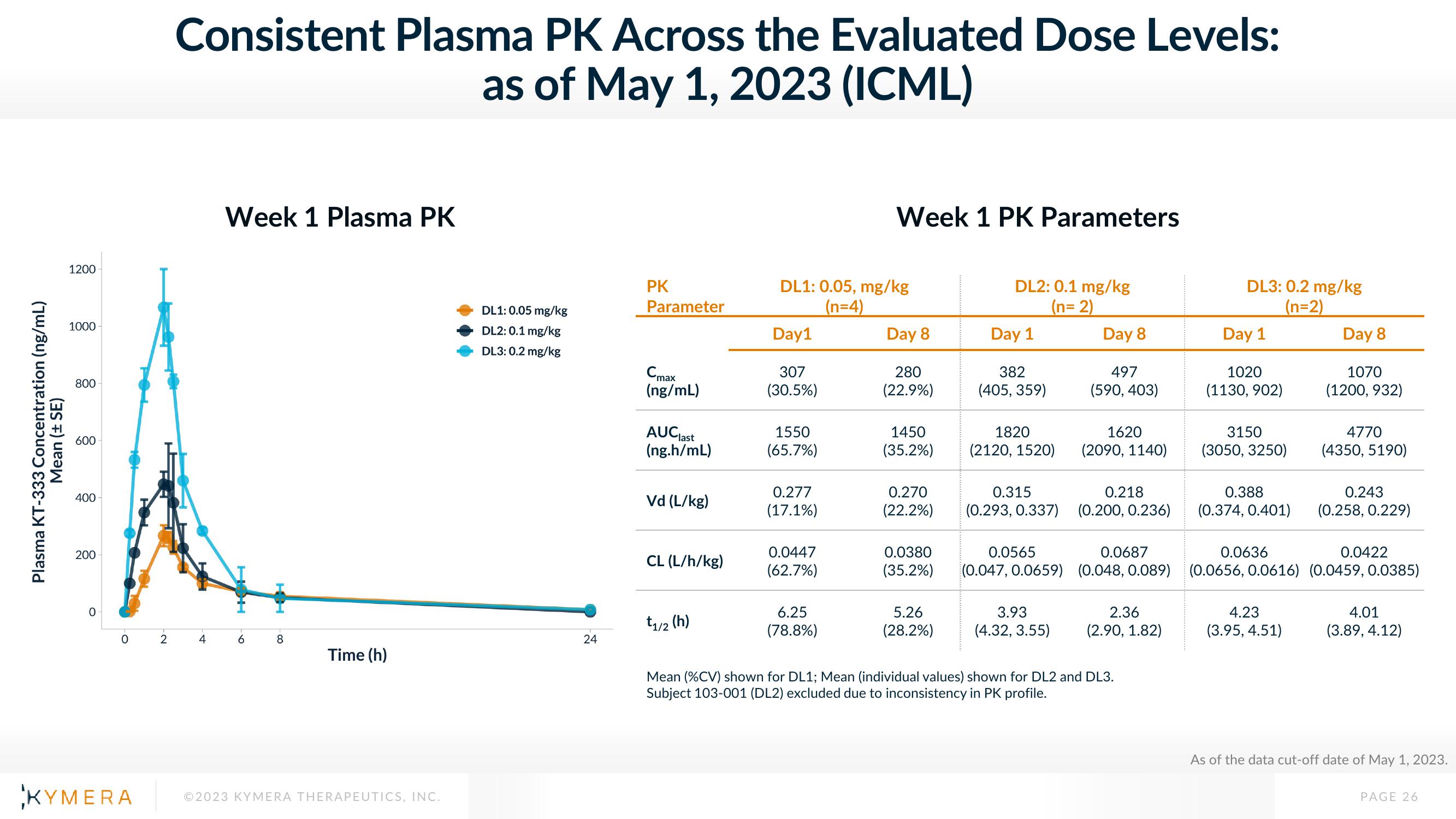
Plasma KT-333 Concentration (ng/mL)
Mean (± SE)
1200
1000-
800
600
400-
200
0
0
KYMERA
2
Consistent Plasma PK Across the Evaluated Dose Levels:
as of May 1, 2023 (ICML)
4
Week 1 Plasma PK
HH
6
HH
8
Time (h)
©2023 KYMERA THERAPEUTICS, INC.
DL1: 0.05 mg/kg
DL2: 0.1 mg/kg
DL3: 0.2 mg/kg
24
PK
Parameter
Cmax
(ng/mL)
AUC last
(ng.h/mL)
Vd (L/kg)
CL (L/h/kg)
t₁/2 (h)
DL1: 0.05, mg/kg
(n=4)
Day1
307
(30.5%)
1550
(65.7%)
0.277
(17.1%)
0.0447
(62.7%)
Week 1 PK Parameters
6.25
(78.8%)
Day 8
280
(22.9%)
1450
(35.2%)
0.270
(22.2%)
0.0380
(35.2%)
5.26
(28.2%)
DL2: 0.1 mg/kg
(n=2)
Day 1
382
(405, 359)
1820
(2120, 1520)
0.315
(0.293, 0.337)
0.0565
(0.047, 0.0659)
3.93
(4.32, 3.55)
Day 8
497
(590, 403)
1620
(2090, 1140)
0.218
(0.200, 0.236)
0.0687
(0.048, 0.089)
2.36
(2.90, 1.82)
Mean (%CV) shown for DL1; Mean (individual values) shown for DL2 and DL3.
Subject 103-001 (DL2) excluded due to inconsistency in PK profile.
DL3: 0.2 mg/kg
(n=2)
Day 1
1020
(1130, 902)
3150
(3050, 3250)
0.388
(0.374, 0.401)
0.0636
(0.0656, 0.0616)
4.23
(3.95, 4.51)
Day 8
1070
(1200, 932)
4770
(4350, 5190)
0.243
(0.258, 0.229)
0.0422
(0.0459, 0.0385)
4.01
(3.89, 4.12)
As of the data cut-off date of May 1, 2023.
PAGE 26View entire presentation