ATAI Investor Presentation Deck
SUMMARY
OWNERSHIP 22.5%¹
PRODUCT
PHARMA-
COLOGY
PRODUCT
FEATURES
INDICATIONS
CURRENT
STATUS
INTELLECTUAL
PROPERTY
HIGHLIGHT
Oral Psilocybin (COMP360)
5-HT2A-R agonist
Rapid onset, potential for sustained efficacy
after single dose
Primary: Treatment Resistant Depression,
Anorexia Nervosa
Potential: Major Depressive Disorder, Autism,
Bipolar Disorder, Chronic Cluster Headache
COMP360 Phase 3 (TRD) program expected to
commence in 4Q 2022
COMP 360 Phase 2 (Anorexia) trial launched
Proprietary formulation of synthetic psilocybin,
COMP360
COMP360 demonstrated efficacy in reducing
depressive symptom severity with rapid and
durable response in Phase 2b study
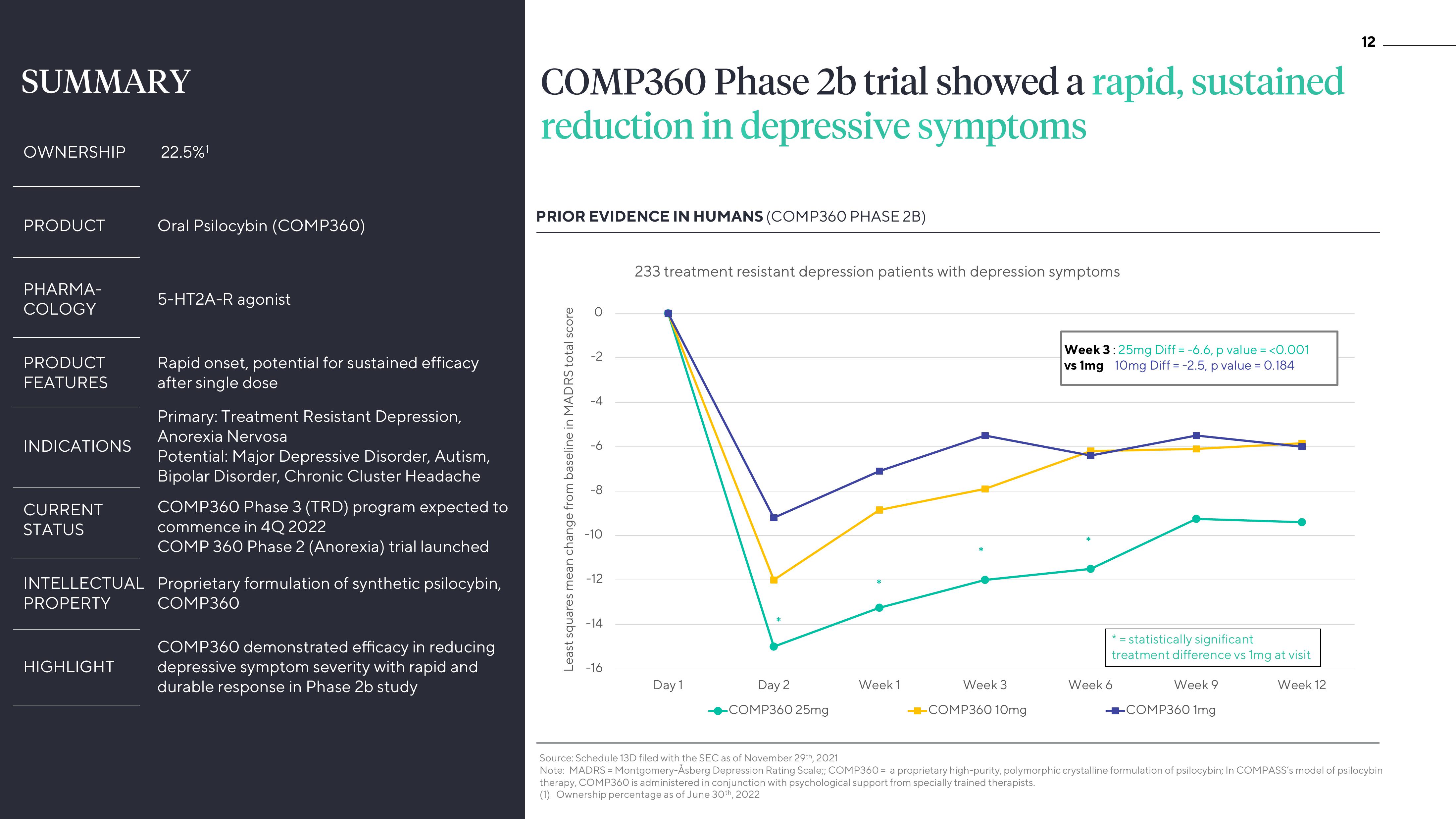
COMP360 Phase 2b trial showed a rapid, sustained
reduction in depressive symptoms
PRIOR EVIDENCE IN HUMANS (COMP360 PHASE 2B)
Least squares mean change from baseline in MADRS total score
O
-8
-10
-12
-14
-16
233 treatment resistant depression patients with depression symptoms
Day 1
Day 2
-COMP360 25mg
Week 1
Week 3
--COMP360 10mg
Week 3:25mg Diff = -6.6, p value = <0.001
vs 1mg 10mg Diff = -2.5, p value = 0.184
* = statistically significant
treatment difference vs 1mg at visit
Week 6
Week 9
COMP360 1mg
Week 12
12
Source: Schedule 13D filed with the SEC as of November 29th, 2021
Note: MADRS = Montgomery-Åsberg Depression Rating Scale;; COMP360 = a proprietary high-purity, polymorphic crystalline formulation of psilocybin; In COMPASS's model of psilocybin
therapy, COMP360 is administered in conjunction with psychological support from specially trained therapists.
(1) Ownership percentage as of June 30th, 2022View entire presentation