Ocuphire Pharma Investor Presentation Deck
APX3330 - Phase 2 Summary and Next Steps
●
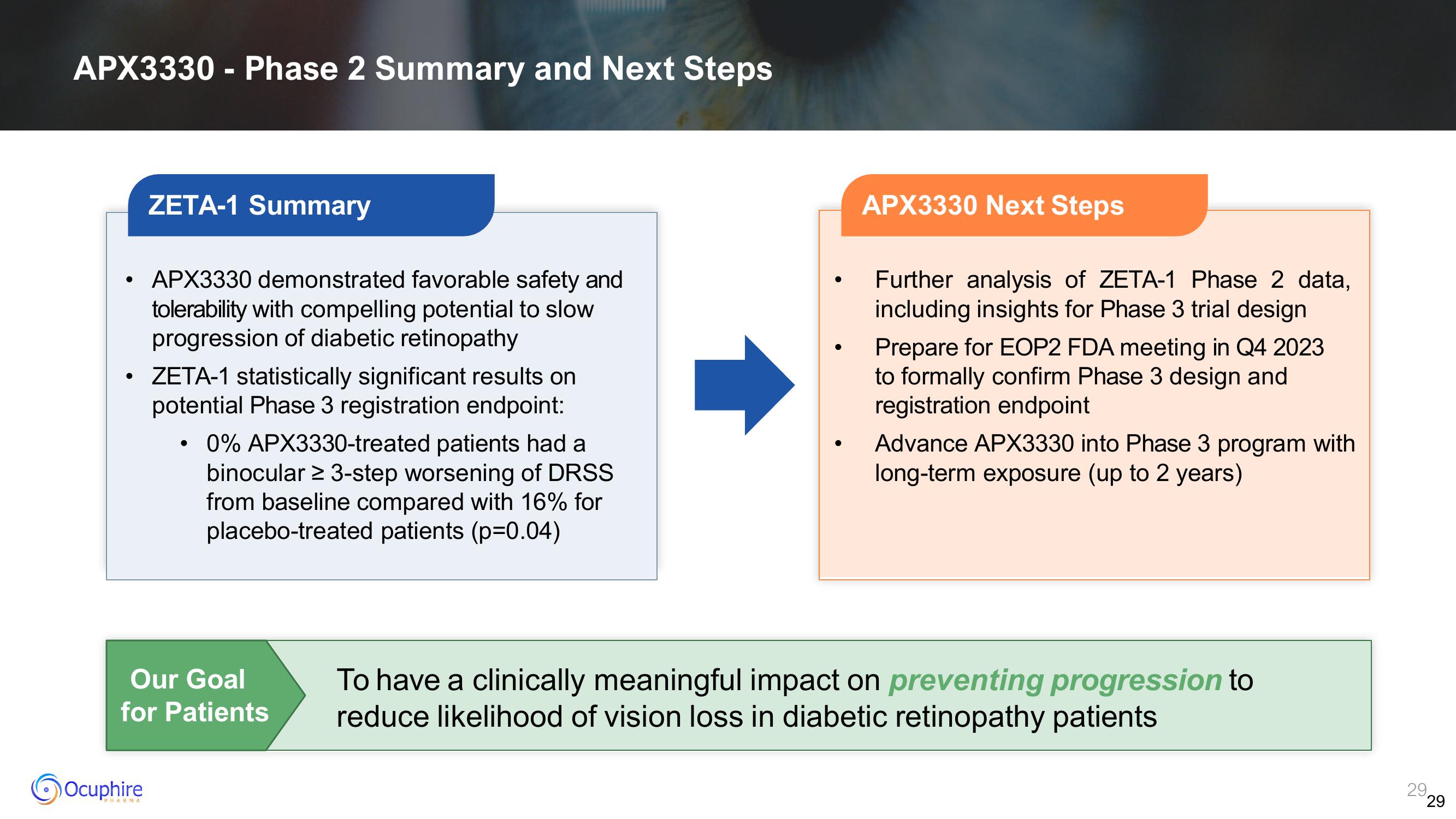
ZETA-1 Summary
Ocuphire
APX3330 demonstrated favorable safety and
tolerability with compelling potential to slow
progression of diabetic retinopathy
ZETA-1 statistically significant results on
potential Phase 3 registration endpoint:
●
0% APX3330-treated patients had a
binocular ≥ 3-step worsening of DRSS
from baseline compared with 16% for
placebo-treated patients (p=0.04)
Our Goal
for Patients
APX3330 Next Steps
Further analysis of ZETA-1 Phase 2 data,
including insights for Phase 3 trial design
Prepare for EOP2 FDA meeting in Q4 2023
to formally confirm Phase 3 design and
registration endpoint
Advance APX3330 into Phase 3 program with
long-term exposure (up to 2 years)
To have a clinically meaningful impact on preventing progression to
reduce likelihood of vision loss in diabetic retinopathy patients
29,
29View entire presentation