Ocuphire Pharma Investor Day Presentation Deck
P
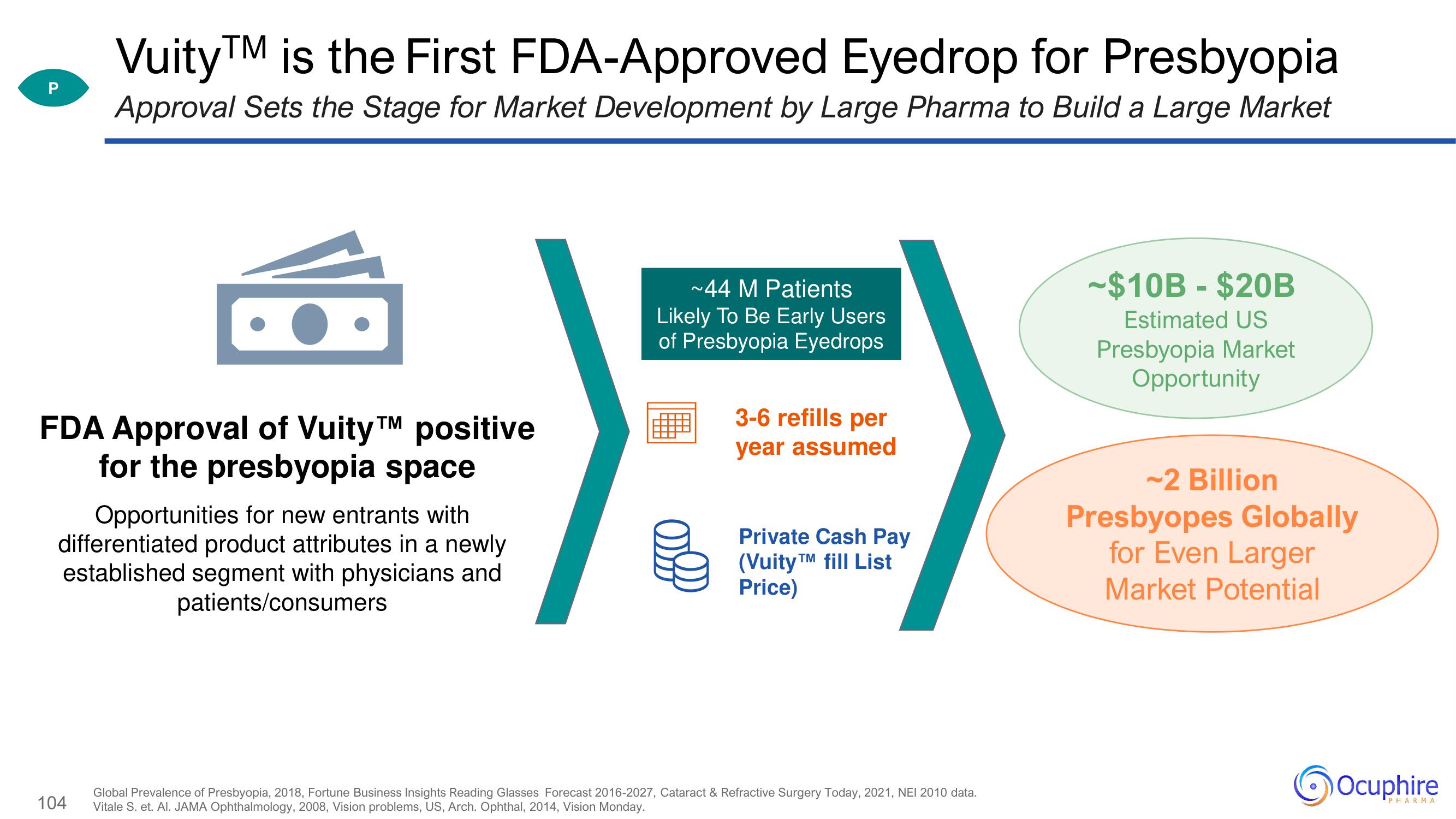
VuityTM is the First FDA-Approved Eyedrop for Presbyopia
Approval Sets the Stage for Market Development by Large Pharma to Build a Large Market
FDA Approval of VuityTM positive
for the presbyopia space
Opportunities for new entrants with
differentiated product attributes in a newly
established segment with physicians and
patients/consumers
104
~44 M Patients
Likely To Be Early Users
of Presbyopia Eyedrops
3-6 refills per
year assumed
Private Cash Pay
(VuityTM fill List
Price)
Global Prevalence of Presbyopia, 2018, Fortune Business Insights Reading Glasses Forecast 2016-2027, Cataract & Refractive Surgery Today, 2021, NEI 2010 data.
Vitale S. et. Al. JAMA Ophthalmology, 2008, Vision problems, US, Arch. Ophthal, 2014, Vision Monday.
-$10B - $20B
Estimated US
Presbyopia Market
Opportunity
~2 Billion
Presbyopes Globally
for Even Larger
Market Potential
Ocuphire
PHARMAView entire presentation