AstraZeneca Results Presentation Deck
Tagrisso and Imfinzi
Global growth boosted by Europe and EMS
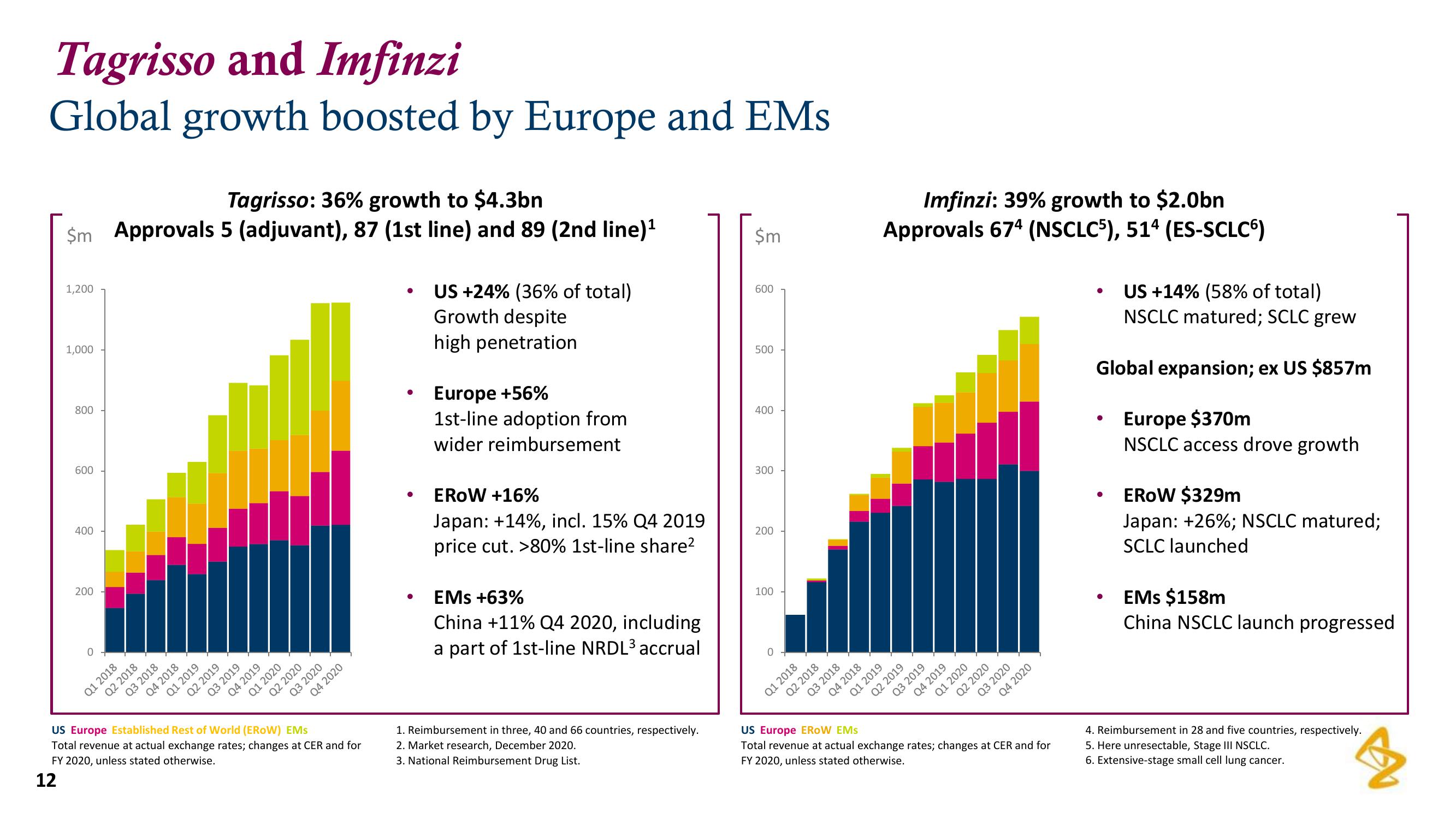
Tagrisso: 36% growth to $4.3bn
$m Approvals 5 (adjuvant), 87 (1st line) and 89 (2nd line)¹
1,200
1,000
800
600
400
200
0
Q1 2018
Q2 2018
Q3 2018
Q4 2018
Q1 2019
Q2 2019
Q3 2019
Q4 2019
Q1 2020
Q2 2020
Q3 2020
Q4 2020
US Europe Established Rest of World (EROW) EMS
Total revenue at actual exchange rates; changes at CER and for
FY 2020, unless stated otherwise.
12
●
●
●
US +24% (36% of total)
Growth despite
high penetration
Europe +56%
1st-line adoption from
wider reimbursement
EROW +16%
Japan: +14%, incl. 15% Q4 2019
price cut. >80% 1st-line share²
EMs +63%
China +11% Q4 2020, including
a part of 1st-line NRDL³ accrual
1. Reimbursement in three, 40 and 66 countries, respectively.
2. Market research, December 2020.
3. National Reimbursement Drug List.
$m
600
500
400
300
200
100
Q1 2018
Q2 2018
Q3
2018
Q4
Imfinzi: 39% growth to $2.0bn
Approvals 674 (NSCLC5), 514 (ES-SCLC6)
2018
Q1 2019
Q2 2019
Q3 2019
2019
Q4
Q1 2020
Q2 2020
Q3 2020
Q4 2020
US Europe
EMS
EROW
Total revenue at actual exchange rates; changes at CER and for
FY 2020, unless stated otherwise.
US +14% (58% of total)
NSCLC matured; SCLC grew
Global expansion; ex US $857m
Europe $370m
NSCLC access drove growth
●
●
EROW $329m
Japan: +26%; NSCLC matured;
SCLC launched
EMs $158m
China NSCLC launch progressed
4. Reimbursement in 28 and five countries, respectively.
5. Here unresectable, Stage III NSCLC.
6. Extensive-stage small cell lung cancer.
3View entire presentation