BenevolentAI Investor Presentation Deck
BEN-8744
Affects 0.4% US population(¹), 1.7 million
patients in 7MM(¹), forecast $7.8bn market by
2026(²)
- Ulcerative Colitis (UC)
• A chronic, lifelong disease that causes inflammation and
ulceration of the inner lining of the colon and rectum
• Efficacy - 20-40% of Moderate-severe patients do not respond to
anti-TNF (main treatment paradigm) (3)
• Safety - Treatments have many side effects from steroids to
anti-TNF and JAK inhibitors (black box warnings) (4)
• High unmet need for an alternative oral small molecule
treatment option with improved safety profile and efficacy in
treatment of refractory patients
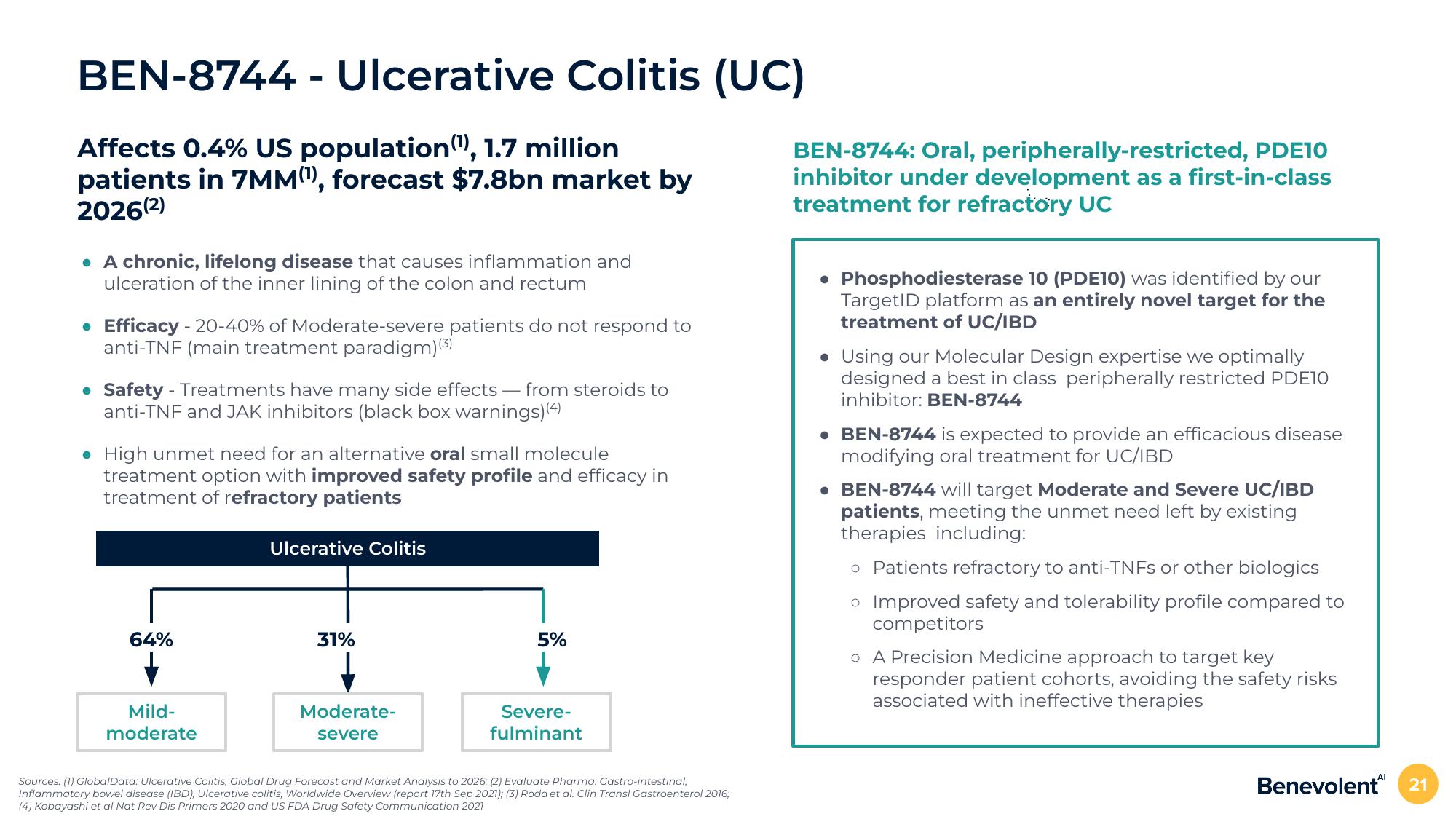
64%
Mild-
moderate
Ulcerative Colitis
31%
Moderate-
severe
5%
Severe-
fulminant
Sources: (1) GlobalData: Ulcerative Colitis, Global Drug Forecast and Market Analysis to 2026; (2) Evaluate Pharma: Gastro-intestinal,
Inflammatory bowel disease (IBD), Ulcerative colitis, Worldwide Overview (report 17th Sep 2021); (3) Roda et al. Clin Transl Gastroenterol 2016;
(4) Kobayashi et al Nat Rev Dis Primers 2020 and US FDA Drug Safety Communication 2021
BEN-8744: Oral, peripherally-restricted, PDE10
inhibitor under development as a first-in-class
treatment for refractory UC
• Phosphodiesterase 10 (PDE10) was identified by our
TargetID platform as an entirely novel target for the
treatment of UC/IBD
• Using our Molecular Design expertise we optimally
designed a best in class peripherally restricted PDE10
inhibitor: BEN-8744
• BEN-8744 is expected to provide an efficacious disease
modifying oral treatment for UC/IBD
• BEN-8744 will target Moderate and Severe UC/IBD
patients, meeting the unmet need left by existing
therapies including:
o Patients refractory to anti-TNFS or other biologics
o Improved safety and tolerability profile compared to
competitors
o A Precision Medicine approach to target key
responder patient cohorts, avoiding the safety risks
associated with ineffective therapies
Benevolent 21View entire presentation