BioAtla Investor Presentation Deck
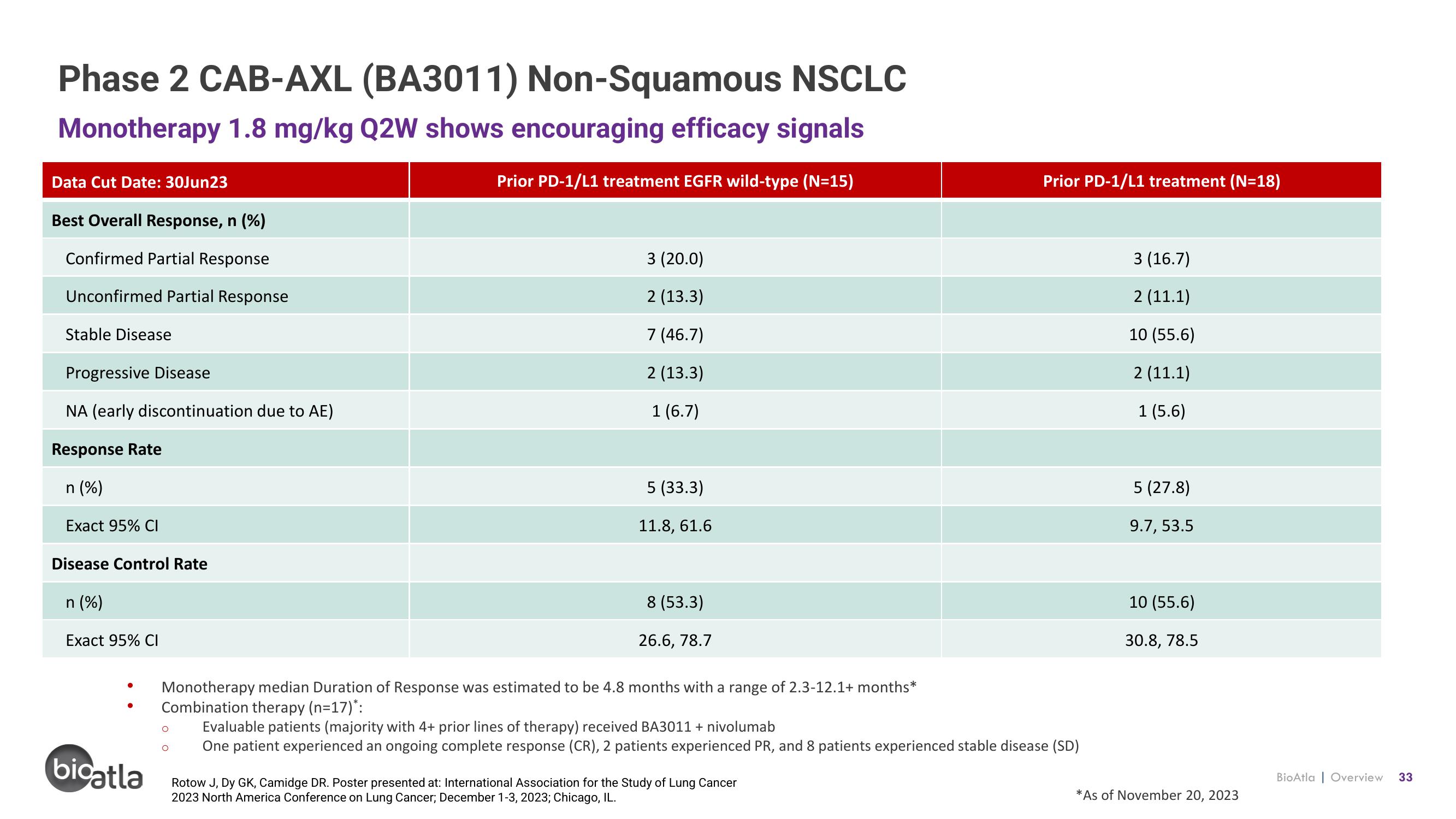
Phase 2 CAB-AXL (BA3011) Non-Squamous NSCLC
Monotherapy 1.8 mg/kg Q2W shows encouraging efficacy signals
Prior PD-1/L1 treatment EGFR wild-type (N=15)
Data Cut Date: 30Jun23
Best Overall Response, n (%)
Confirmed Partial Response
Unconfirmed Partial Response
Stable Disease
Progressive Disease
NA (early discontinuation due to AE)
Response Rate
n (%)
Exact 95% CI
Disease Control Rate
n (%)
Exact 95% CI
bicatla
3 (20.0)
2 (13.3)
7 (46.7)
2 (13.3)
1 (6.7)
5 (33.3)
11.8, 61.6
8 (53.3)
26.6, 78.7
Monotherapy median Duration of Response was estimated to be 4.8 months with a range of 2.3-12.1+ months*
Combination therapy (n=17)*:
Prior PD-1/L1 treatment (N=18)
Evaluable patients (majority with 4+ prior lines of therapy) received BA3011 + nivolumab
One patient experienced an ongoing complete response (CR), 2 patients experienced PR, and 8 patients experienced stable disease (SD)
Rotow J, Dy GK, Camidge DR. Poster presented at: International Association for the Study of Lung Cancer
2023 North America Conference on Lung Cancer; December 1-3, 2023; Chicago, IL.
3 (16.7)
2 (11.1)
10 (55.6)
2 (11.1)
1 (5.6)
5 (27.8)
9.7, 53.5
10 (55.6)
30.8, 78.5
*As of November 20, 2023
BioAtla| Overview 33View entire presentation