BioNTech Investor Day Presentation Deck
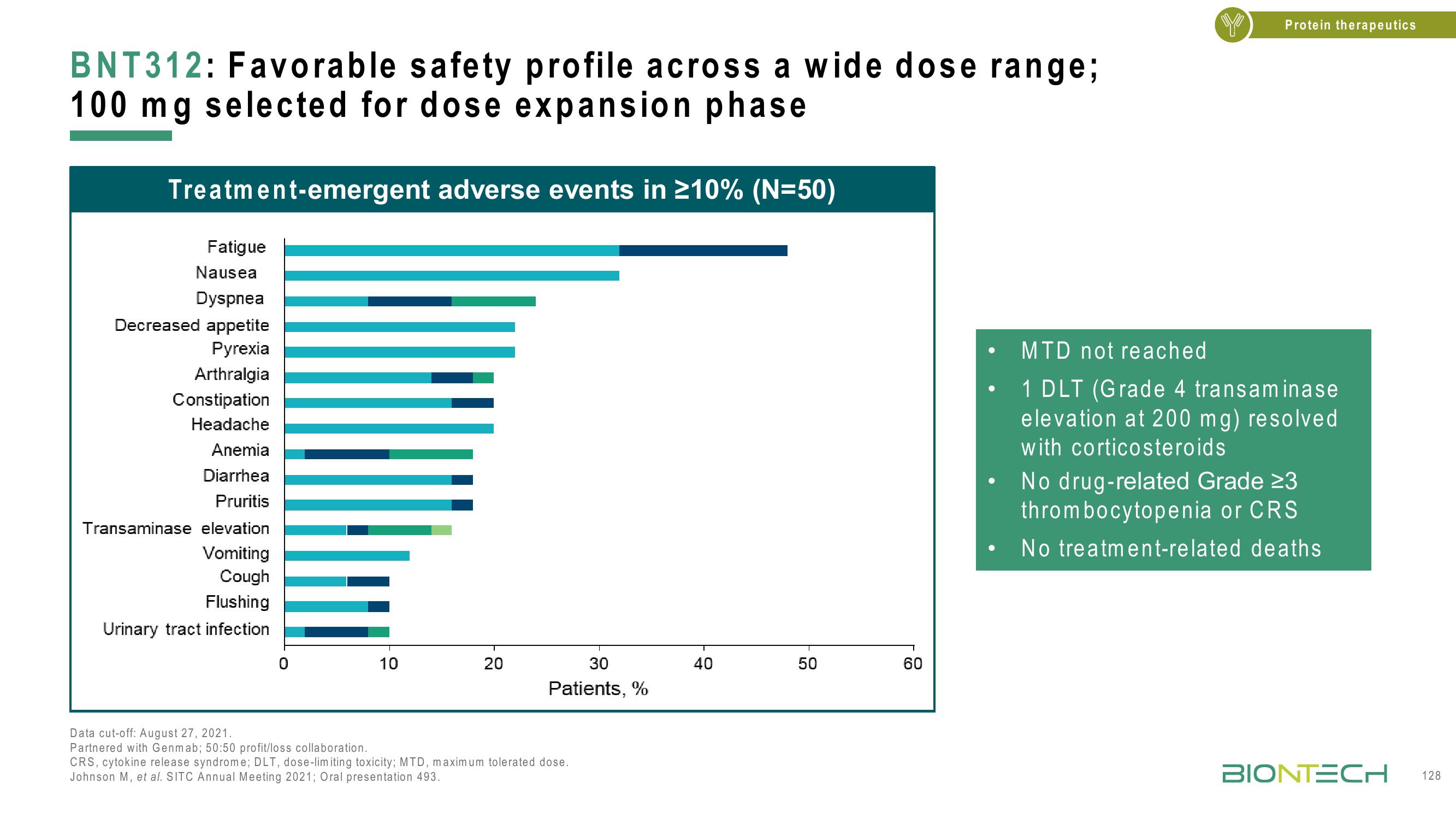
BNT312: Favorable safety profile across a wide dose range;
100 mg selected for dose expansion phase
Treatment-emergent adverse events in ≥10% (N=50)
Fatigue
Nausea
Dyspnea
Decreased appetite
Pyrexia
Arthralgia
Constipation
Headache
Anemia
Diarrhea
Pruritis
Transaminase elevation
Vomiting
Cough
Flushing
Urinary tract infection
0
10
T
20
30
Patients, %
Data cut-off: August 27, 2021.
Partnered with Genmab; 50:50 profit/loss collaboration.
CRS, cytokine release syndrome; DLT, dose-limiting toxicity; MTD, maximum tolerated dose.
Johnson M, et al. SITC Annual Meeting 2021; Oral presentation 493.
T
40
50
60
Protein therapeutics
MTD not reached
1 DLT (Grade 4 transaminase
elevation at 200 mg) resolved
with corticosteroids
No drug-related Grade 23
thrombocytopenia or CRS
No treatment-related deaths
BIONTECH 128View entire presentation