Immix Biopharma Investor Presentation Deck
N-GENIUS Platform Process
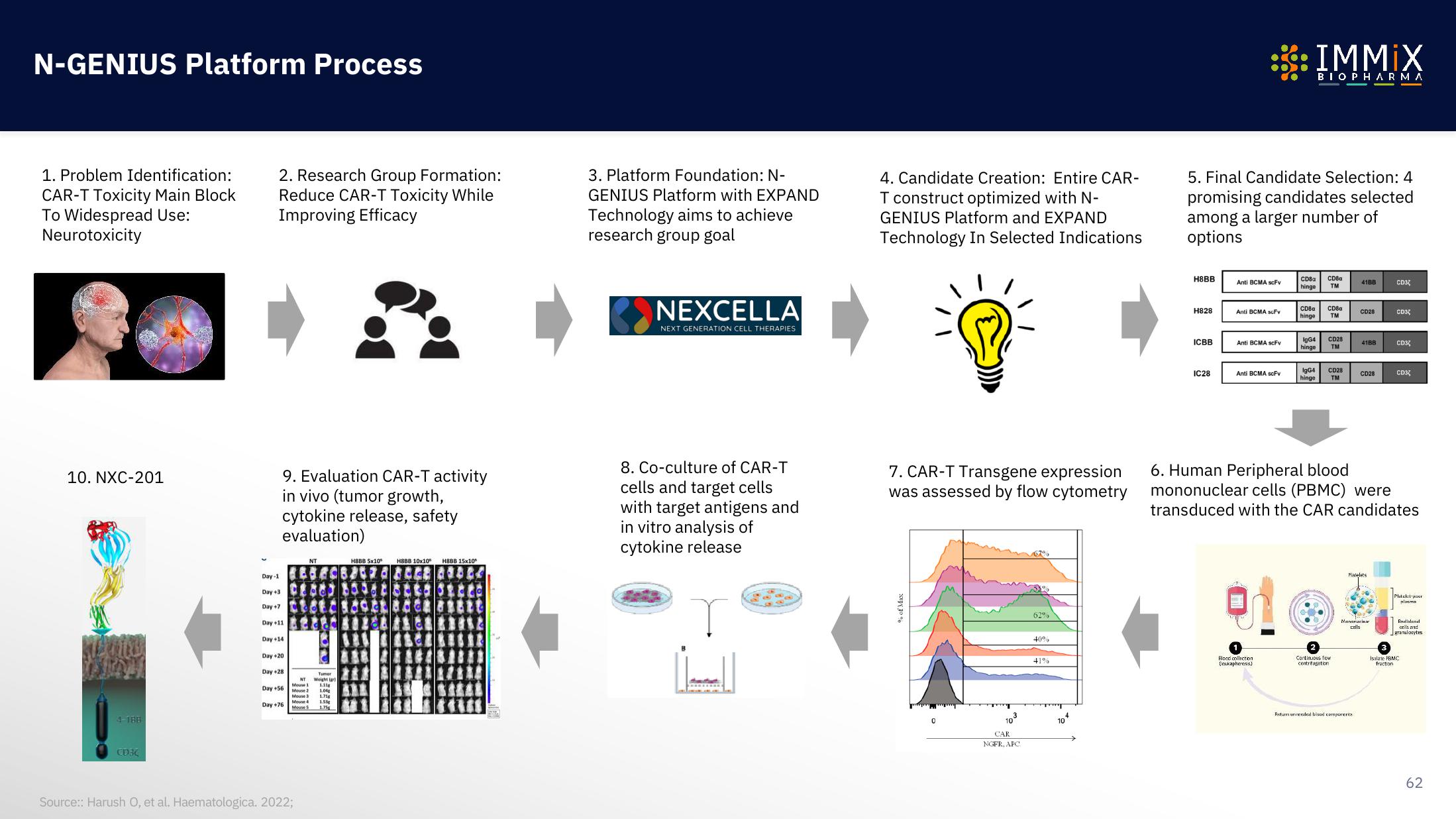
1. Problem Identification:
CAR-T Toxicity Main Block
To Widespread Use:
Neurotoxicity
10. NXC-201
4-188
CD3
2. Research Group Formation:
Reduce CAR-T Toxicity While
Improving Efficacy
Day-1
Day +3
Day +7
9. Evaluation CAR-T activity
in vivo (tumor growth,
cytokine release, safety
evaluation)
Day +11
Day +14
Day +20
Day +28
Day +56
Day +76
NT
Mouse 1
Mouse 2
NT
Mouse 3
Mouse 4
Source:: Harush O, et al. Haematologica. 2022;
Tumor
1.04g
171g
1.50
HBBB 5x10
za
HBBB 10x10 H8BB 15x105
3. Platform Foundation: N-
GENIUS Platform with EXPAND
Technology aims to achieve
research group goal
NEXCELLA
NEXT GENERATION CELL THERAPIES
8. Co-culture of CAR-T
cells and target cells
with target antigens and
in vitro analysis of
cytokine release
www.
4. Candidate Creation: Entire CAR-
T construct optimized with N-
GENIUS Platform and EXPAND
Technology In Selected Indications
7. CAR-T Transgene expression
was assessed by flow cytometry
% of Max
0
10³
CAR
NGFR, APC
67%
62%
40%
41%
T™
10*
H8BB
5. Final Candidate Selection: 4
promising candidates selected
among a larger number of
options
H828
ICBB
IC28
●●●
S
Anti BCMA scFv
Anti BCMA scFv
Anti BCMA scFv
Anti BCMA scFv
Blood collection
(cukapheress)
IMMİX
BIOPHARMA
CD8a CD8a
hinge
TM
CD8a
hinge
CD8a
TM
IgG4 CD28
hinge TM
IgG4 CD28
hinge TM
Continuous flow
centrifugation
41BB
CD28
Retum unneeded blood components
41BB
CD28
Manarucles
cels
CD3
6. Human Peripheral blood
mononuclear cells (PBMC) were
transduced with the CAR candidates
CD3
CD3
CD3
Plack por
plasma
Endalend
cells and
granulocytes
Isalate PBMC
fraction
62View entire presentation