BioAtla Investor Presentation Deck
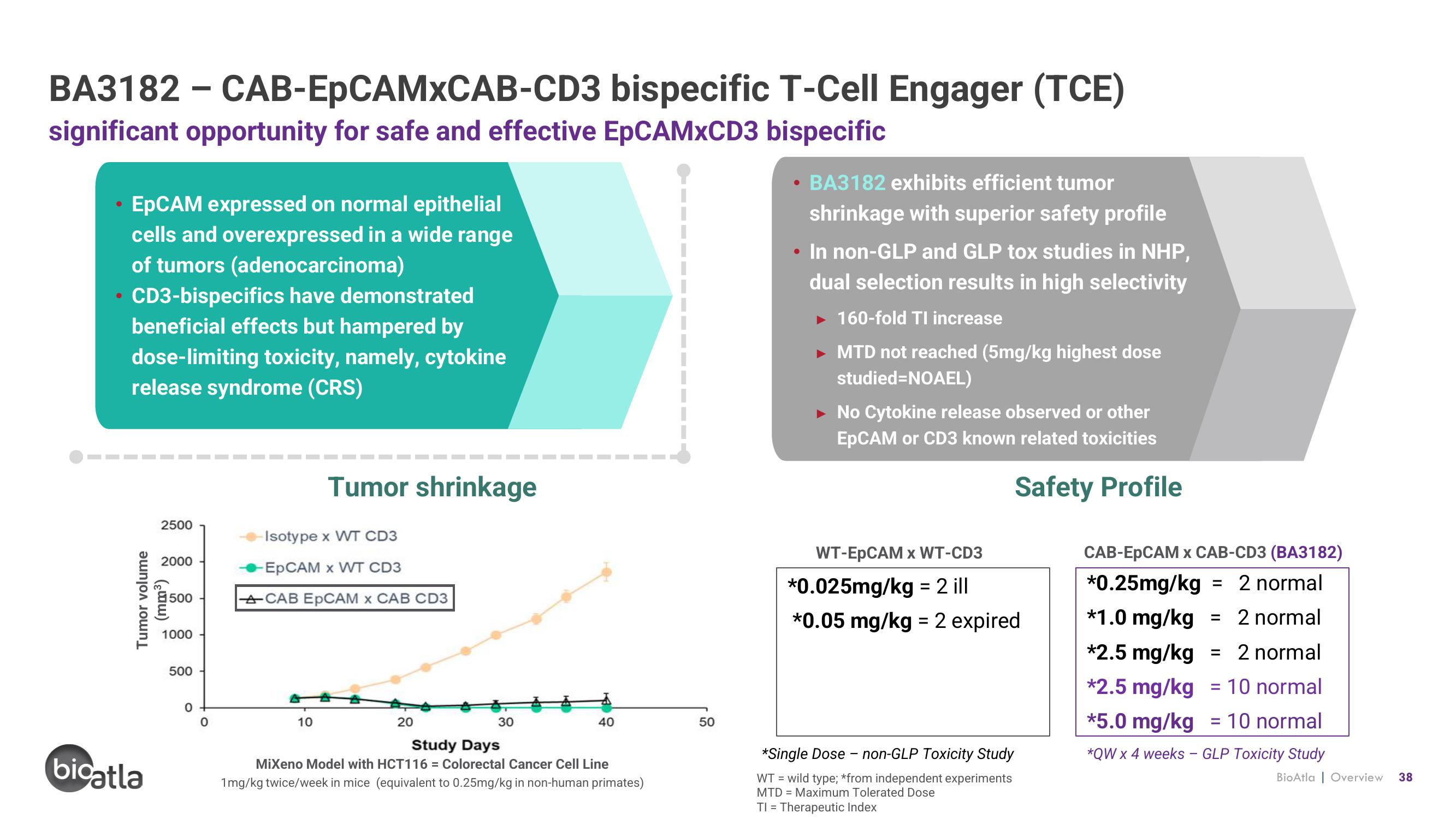
BA3182 - CAB-EpCAMxCAB-CD3
significant opportunity for safe and effective EpCAMXCD3 bispecific
EpCAM expressed on normal epithelial
cells and overexpressed in a wide range
of tumors (adenocarcinoma)
CD3-bispecifics have demonstrated
beneficial effects but hampered by
dose-limiting toxicity, namely, cytokine
release syndrome (CRS)
2500
Tumor volume
(mm³)
bicatla
2000
1500
1000
500
0
0
Tumor shrinkage
Isotype x WT CD3
-EpCAM x WT CD3
ACAB EpCAM x CAB CD3
10
20
bispecific T-Cell Engager (TCE)
30
40
Study Days
MiXeno Model with HCT116= Colorectal Cancer Cell Line
1mg/kg twice/week in mice (equivalent to 0.25mg/kg in non-human primates)
50
●
BA3182 exhibits efficient tumor
shrinkage with superior safety profile
• In non-GLP and GLP tox studies in NHP,
dual selection results in high selectivity
► 160-fold TI increase
MTD not reached (5mg/kg highest dose
studied=NOAEL)
No Cytokine release observed or other
EpCAM or CD3 known related toxicities
Safety Profile
WT-EpCAM x WT-CD3
*0.025mg/kg = 2 ill
*0.05 mg/kg = 2 expired
*Single Dose - non-GLP Toxicity Study
WT = wild type; *from independent experiments
MTD Maximum Tolerated Dose
TI = Therapeutic Index
CAB-EpCAM x CAB-CD3 (BA3182)
*0.25mg/kg = 2 normal
*1.0 mg/kg = 2 normal
*2.5 mg/kg = 2 normal
*2.5 mg/kg = 10 normal
*5.0 mg/kg 10 normal
*QW x 4 weeks - GLP Toxicity Study
BioAtla| Overview
=
38View entire presentation